Abstract.
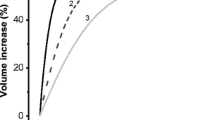
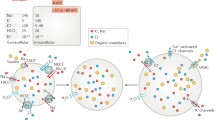
The aim of this study was to investigate swelling-activated taurine and K+ transport in human cervical cancer cells under various culture conditions, testing the hypothesis that the progression of cell cycle was accompanied by differential activities of swelling-activated transport pathways. Aphidicolin, an inhibitor of deoxyribonucleic acid (DNA) synthesis, was used to synchronize the cell cycle. The distribution of cell cycle stage was determined by fluorescence-activated cell sorting (FACS). Hypotonicity activated taurine efflux, which was sensitive to tamoxifen and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB). Cell swelling also induced both Cl–-dependent and -independent K+ (86Rb+) efflux, presumably mediated by KCl cotransport (KCC) and Ca2+-activated K+ channels, respectively. Cell cycle arrest in G0/G1 was accompanied by a remarkable decrease in the rate constant for swelling-activated taurine efflux, from 0.20±0.007 to 0.026±0.002 min–1 (n=6). The activity of swelling-activated taurine efflux recovered progressively on re-entry into the cell cycle. After removal of aphidicolin and culture with 10% fetal calf serum for 10 h, the rate constant increased significantly from 0.026±0.002 to 0.093±0.002 min–1 (n=6). After 24 h release from aphidicolin, the efflux rate constant had increased further to 0.195±0.006 min–1 (n=6), a value not significantly different from that in normally proliferating cells. The differential activities of swelling-activated taurine transport matched well with our previous study showing a volume-sensitive anion channel associated with cell cycle progression. In contrast to the differential activities of swelling-activated taurine transport, swelling-activated K+ (86Rb+) transport was independent of the progression of cell cycle. Most importantly, pharmacological blockade of swelling-activated taurine efflux by tamoxifen or NPPB caused proliferating cervical cancer cells to arrest in G0/G1, suggesting that the activity of this efflux was associated with G1/S checkpoint progression. This study provides new and important information on the functional significance of swelling-activated transport system in the regulation of cell cycle clock of human cervical cancer cells.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received after revision: 11 October 2000
Electronic Publication
Rights and permissions
About this article
Cite this article
Shen, MR., Chou, CY. & Ellory, J. Swelling-activated taurine and K+ transport in human cervical cancer cells: association with cell cycle progression. Pflügers Arch - Eur J Physiol 441, 787–795 (2001). https://doi.org/10.1007/s004240000476
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004240000476