Abstract
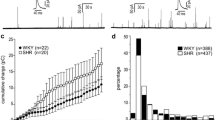
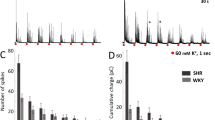
The hypersecretory phenotype of adrenal chromaffin cells (CCs) from early spontaneously hypertensive rats (SHRs) mainly results from enhanced Ca2+-induced Ca2+-release (CICR). A key question is if these abnormalities can be traced to the prehypertensive stage. Spontaneous and stimulus-induced catecholamine exocytosis, intracellular Ca2+ signals, and dense-core granule size and density were examined in CCs from prehypertensive and hypertensive SHRs and compared with age-matched Wistar-Kyoto rats (WKY). During the prehypertensive stage, the depolarization-elicited catecholamine exocytosis was ~ 2.9-fold greater in SHR than in WKY CCs. Interestingly, in half of CCs the exocytosis was indistinguishable from WKY CCs, while it was between 3- and sixfold larger in the other half. Likewise, caffeine-induced exocytosis was ~ twofold larger in prehypertensive SHR. Accordingly, depolarization and caffeine application elicited [Ca2+]i rises ~ 1.5-fold larger in prehypertensive SHR than in WKY CCs. Ryanodine reduced the depolarization-induced secretion in prehypertensive SHR by 57%, compared to 14% in WKY CCs, suggesting a greater contribution of intracellular Ca2+ release to exocytosis. In SHR CCs, the mean spike amplitude and charge per spike were significantly larger than in WKY CCs, regardless of age and stimulus type. This difference in granule content could explain in part the enhanced exocytosis in SHR CCs. However, electron microscopy did not reveal significant differences in granule size between SHRs and WKY rats’ adrenal medulla. Nonetheless, preSHR and hypSHR display 63% and 82% more granules than WKY, which could explain in part the enhanced catecholamine secretion. The mechanism responsible for the heterogeneous population of prehypertensive SHR CCs and the bias towards secreting more medium and large granules remains unexplained.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Albillos A, Dernick G, Horstmann H, Almers W, de Toledo GA, Lindau M (1997) The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389(6650):509–512. https://doi.org/10.1038/39081
Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, García AG, García-Sancho J, Montero M, Alvarez J (1999) Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol 144(2):241–254. https://doi.org/10.1083/jcb.144.2.241
Anderson EA, Sinkey CA, Lawton WJ, Mark AL (1989) Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14(2):177–183. https://doi.org/10.1161/01.HYP.14.2.177
Aoki K, Takikawa K, Hotta K (1973) Role of adrenal cortex and medulla in hypertension. Nat New Biol 241(108):122–123. https://doi.org/10.1038/newbio241122a0
Bhalla A, Chicka MC, Chapman ER (2008) Analysis of the synaptotagmin family during reconstituted membrane fusion. J Biol Chem 283(31):21799–21807. https://doi.org/10.1074/jbc.M709628200
Borkowski KR, Quinn P (1983) The effect of bilateral adrenal demedullation on vascular reactivity and blood pressure in spontaneously hypertensive rats. Br J Pharmacol 80(3):429–437. https://doi.org/10.1111/j.1476-5381.1983.tb10712.x
Carbone E, Borges R, Eiden LE, García AG, Hernández-Cruz A (2019) Chromaffin cells of the adrenal medulla: physiology, pharmacology, and disease. Compr Physiol 9(4):1443–1502. https://doi.org/10.1002/cphy.c190003
de Champlain J, Farley L, Cousineau D, van Ameringen MR (1976) Circulating catecholamine levels in human and experimental hypertension. Circ Res 38(2):109–114. https://doi.org/10.1161/01.RES.38.2.109
Chapman ER (2008) How does synaptotagmin trigger neurotransmitter release? Ann Rev Biochem 77(1):615–641. https://doi.org/10.1146/annurev.biochem.77.062005.101135
Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OHL (1995) Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 91(1):161–170. https://doi.org/10.1161/01.CIR.91.1.161
Currie G, Freel EM, Perry CG, Dominiczak AF (2012) Disorders of blood pressure regulation—role of catecholamine biosynthesis, release, and metabolism. Curr Hypertens Rep 14(1):38–45. https://doi.org/10.1007/s11906-011-0239-2
Esler M (2000) The sympathetic system and hypertension. Am J Hypertens 13(6):S99–S105. https://doi.org/10.1016/S0895-7061(00)00225-9
Esler M, Rumantir M, Kaye D, Jennings G, Hastings J, Socratous F, Lambert G (2001) Sympathetic nerve biology In Essential hypertension. Clin Exp Pharmacol Physiol 28(12):986–989. https://doi.org/10.1046/j.1440-1681.2001.03566.x
Finnegan JM, Pihel K, Cahill PS, Huang L, Zerby SE, Ewing AG, Kennedy RT, Wightman RM (1996) Vesicular quantal size measured by amperometry at chromaffin, mast, pheochromocytoma, and pancreatic beta-cells. J Neurochem 66(5):1914–1923. https://doi.org/10.1046/j.1471-4159.1996.66051914.x
Flaa A, Mundal HH, Eide I, Kjeldsen S, Rostrup M (2006) Sympathetic activity and cardiovascular risk factors in young men in the low, normal, and high blood pressure ranges. Hypertension. 47(3):396–402. https://doi.org/10.1161/01.HYP.0000203952.27988.79
Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A (2004) Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem 279(50):52677–52684. https://doi.org/10.1074/jbc.M409241200
Fukuda M, Kowalchyk JA, Zhang X, Martin TFJ, Mikoshiba K (2002) Synaptotagmin IX Regulates Ca2+-dependent Secretion in PC12 Cells. J Biol Chem 277(7):4601–4604. https://doi.org/10.1074/jbc.C100588200
Hernández-Cruz A, Díaz-Muñoz M, Gómez-Chavarín M, Cañedo-Merino R, Protti DA, Escobar AL, Sierralta J, Suárez-Isla BA (1995) Properties of the ryanodine-sensitive release channels that underlie caffeine-induced Ca2+ mobilization from intracellular stores in mammalian sympathetic neurons. Eur J Neurosci 7(8):1684–1699. https://doi.org/10.1111/j.1460-9568.1995.tb00690.x
Hernández-Cruz A, Escobar AL, Jiménez N (1997) Ca2+-induced Ca2+ release phenomena in mammalian sympathetic neurons are critically dependent on the Rate of rise of trigger Ca2+. J Gen Physiol 109(2):147–167. https://doi.org/10.1085/jgp.109.2.147
Jablonskis LT, Howe PRC (1994) Elevated plasma adrenaline in spontaneously hypertensive rats. Blood Pressure 3(1–2):106–111. https://doi.org/10.3109/08037059409101529
Judy WV, Farrell SK (1979) Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension. 1(6):605–614
Kiprov D (1980) Experimental models of hypertension. Cor Et Vasa. 22(1–2):116–128
Kuba K (1994) Ca2+-induced Ca2+ Release in Neurones. Jpn J Physiol 44(6):613–650. https://doi.org/10.2170/jjphysiol.44.613
Lee RM, Borkowski KR, Leenen FH, Tsoporis J, Coughlin M (1991) Combined effect of neonatal sympathectomy and adrenal demedullation on blood pressure and vascular changes in spontaneously hypertensive rats. Circ Res 69(3):714–721. https://doi.org/10.1161/01.RES.69.3.714
Lee RM, Triggle CR, Cheung DW, Coughlin MD (1987) Structural and functional consequence of neonatal sympathectomy on the blood vessels of spontaneously hypertensive rats. Hypertension (Dallas, Tex.: 1979) 10(3):328–338. https://doi.org/10.1161/01.hyp.10.3.328
Li D, Lee C, Buckler K, Parekh A, Herring N, Paterson DJ (2012) Abnormal intracellular calcium homeostasis in sympathetic neurons from young prehypertensive rats. Hypertension 59(3):642–649. https://doi.org/10.1161/HYPERTENSIONAHA.111.186460
Lundin SA, Hallback-Nordlander M (1980) Background of hyperkinetic circulatory state in young spontaneously hypertensive rats. Cardiovasc Res 14(10):561–567. https://doi.org/10.1093/cvr/14.10.561
Malgaroli A, Fesce R, Meldolesi J (1990) Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem 265(6):3005–3008
Miranda-Ferreira R, de Pascual R, Caricati-Neto A, Gandía L, Jurkiewicz A, García AG (2009) Role of the endoplasmic reticulum and mitochondria on quantal catecholamine release from chromaffin cells of control and hypertensive rats. J Pharmacol Exp Ther 329(1):231–240. https://doi.org/10.1124/jpet.108.147413
Miranda-Ferreira R, de Pascual R, de Diego AMG, Caricati-Neto A, Gandía L, Jurkiewicz A, García AG (2008) Single-vesicle catecholamine release has greater quantal content and faster kinetics in chromaffin cells from hypertensive, as compared with normotensive, rats. J Pharmacol Exp Ther 324(2):685–693. https://doi.org/10.1124/jpet.107.128819
Morrison SF, Whitehorn D (1984) Enhanced preganglionic sympathetic nerve responses in spontaneously hypertensive rats. Brain Res 296(1):152–155. https://doi.org/10.1016/0006-8993(84)90522-5
Mosharov EV, Sulzer D (2005) Analysis of exocytotic events recorded by amperometry. Nat Methods. 2(9):651–658. https://doi.org/10.1038/nmeth782
Musial DC, Bomfim GH, Arranz-Tagarro JA, Méndez-López I, Miranda-Ferreira R, Jurkiewicz A, Jurkiewicz NH, García AG, Padín JF (2017) Altered mitochondrial function, calcium signaling, and catecholamine release in chromaffin cells of diabetic and SHR rats. Eur J Pharmacol 815:416–426. https://doi.org/10.1016/j.ejphar.2017.09.045
Okamoto K, Aoki K (1963) Development of a Strain of Spontaneously Hypertensive Rats. Jpn Circ J 27(3):282–293. https://doi.org/10.1253/jcj.27.282
Padín JF, de Diego AMG, Fernández-Morales JC, Merino C, Maroto M, Calvo-Gallardo E, Arranz JA, Yáñez M, García AG (2012) Resveratrol augments nitric oxide generation and causes store calcium release in chromaffin cells. Eur J Pharmacol 685(1–3):99–107. https://doi.org/10.1016/j.ejphar.2012.03.040
Pak CH (1981) Plasma adrenaline and noradrenaline concentrations of the spontaneously hypertensive rat. Jpn Heart J 22(6):987–995. https://doi.org/10.1536/ihj.22.987
Pinto Y (1998) Lessons from rat models of hypertension from Goldblatt to genetic engineering. Cardiovasc Res 39(1):77–88. https://doi.org/10.1016/S0008-6363(98)00077-7
Sakaguchi A, LeDoux JE, Reis DJ (1983) Sympathetic nerves and adrenal medulla: contributions to cardiovascular-conditioned emotional responses in spontaneously hypertensive rats. Hypertension. 5(5):728–738. https://doi.org/10.1161/01.HYP.5.5.728
Segura-Chama P, López-Bistrain P, Pérez-Armendáriz EM, Jiménez-Pérez N, Millán-Aldaco D, Hernández-Cruz A (2015) Enhanced Ca2+-induced Ca2+ release from intracellular stores contributes to catecholamine hypersecretion in adrenal chromaffin cells from spontaneously hypertensive rats. Pflügers Archiv - Eur J Physiol 467(11):2307–2323. https://doi.org/10.1007/s00424-015-1702-8
Shamanaev AY, Aliev OI, Anishchenko AM, Sidekhmenova AV, Plotnikov MB (2016) Specificity of the Hemodynamic Indices’ Shift in SHR Line Rats at Different Age. Ontogenez 47(5):320–323
Shanks J, Manou-Stathopoulou S, Lu C-J, Li D, Paterson DJ, Herring N (2013) Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. Am J Physiol-Heart Circ Physiol 305(7):H980–H986. https://doi.org/10.1152/ajpheart.00255.2013
Sietzen M, Schober M, Fischer-Colbrie R, Scherman D, Sperk G, Winkler H (1987) Rat adrenal medulla: Levels of chromogranins, enkephalins, dopamine β-hydroxylase and of the amine transporter are changed by nervous activity and hypophysectomy. Neuroscience 22(1):131–139. https://doi.org/10.1016/0306-4522(87)90203-X
Sutko JI, Airey JA, Welch W, Ruest L (1997) The pharmacology of ryanodine and related compounds. Am Soc Pharmacol Exp Ther 49:1
Tischler AS, Ruzicka LA, DeLellis RA (1991) Regulation of neurotensin content in adrenal medullary cells: comparison of PC12 cells to normal rat chromaffin cells in vitro. Neuroscience 43(2–3):671–678. https://doi.org/10.1016/0306-4522(91)90325-I
Tischler AS, Ziar J, Downing JC, Mcclain RM (1995) Sustained stimulation of rat adrenal chromaffin cell proliferation by reserpine. Toxicol Appl Pharmacol 135(2):254–257. https://doi.org/10.1006/taap.1995.1231
Vavřínová A, Behuliak M, Bencze M, Vaněčková I, Zicha J (2019) Which sympathoadrenal abnormalities of adult spontaneously hypertensive rats can be traced to a prehypertensive stage? Hypertens Res 42(7):949–959. https://doi.org/10.1038/s41440-018-0198-y
Vavřínová A, Behuliak M, Bencze M, Vodička M, Ergang P, Vaněčková I, Zicha J (2019) Sympathectomy-induced blood pressure reduction in adult normotensive and hypertensive rats is counteracted by enhanced cardiovascular sensitivity to vasoconstrictors. Hypertens Res 42(12):1872–1882. https://doi.org/10.1038/s41440-019-0319-2
Vites AM, Pappano J (1994) Distinct modes of inhibition by ruthenium red and ryanodine of calcium-induced calcium release in avian atrium. J Pharmacol Exp Ther 268(3):1476–1484
Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Viveros OH (1991) Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci 88:10754–10758
Zhang Z, Wu Y, Wang Z, Dunning FM, Rehfuss J, Ramanan D, Chapman ER, Jackson MB (2011) Release mode of large and small dense-core vesicles specified by different synaptotagmin isoforms in PC12 cells. Mol Biol Cell 22(13):2324–2336. https://doi.org/10.1091/mbc.e11-02-0159
Acknowledgements
The authors wish to thank Drs. Citlali Trueta and Fredy Cifuentes for discussions and advice, MVZ Claudia V. Rivera-Cerecedo for expert animal breeding, and Francisco Perez for expert computing assistance. Peña del Castillo J. G. is a Ph. D. student from the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received the fellowship #306030 from CONACyT. This study was conducted as part of the requirements to obtain her Ph. D. Supported by Grants 315803 and 314839 to A-HC from Consejo Nacional de Ciencia y Tecnología (CONACYT), México and PAPIIT AG200119 from Dirección General de Asuntos del Personal Académico (DGAPA-UNAM). Also supported by a grant from Ministerio de Ciencia e Innovación (Project PID2020-114824GB-I00 to LMG).
Funding
This study is financially supported by Grants 315803 (Laboratorio Nacional de Canalopatías) from Consejo Nacional de Ciencia y Tecnología (CONACYT), México, and PAPIIT AG200119 from Dirección General de Asuntos del Personal Académico (DGAPA-UNAM) and by a grant of Ministerio de Ciencia e Innovación (Project PID2020-114824GB-I00 to LMG).
Author information
Authors and Affiliations
Contributions
Peña-del Castillo performed the experiments, analyzed data, prepared figures, and wrote the paper, Segura Chama performed research and analyzed data, Rincón-Heredia analyzed data, Diana Millán-Aldaco performed research, contributed new methods, Yolanda Giménez-Molina, José Villanueva, and Luis Miguel Gutiérrez, contributed new method and analyzed data. Arturo Hernández-Cruz conceived the study, wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Research involving animals complies with the Mexican Guide’s guidelines for the Care and Use of Laboratory Animals of the Secretary of Agriculture (SAGARPA NOM-062-Z00–1999). The Institutional Committee of Care and Use of Laboratory Animals (CICUAL-IFC) approved the experimental protocols used here (protocol AHC24-141). Research does not involve human patients.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peña del Castillo, J.G., Segura-Chama, P., Rincón-Heredia, R. et al. Development of the hypersecretory phenotype in the population of adrenal chromaffin cells from prehypertensive SHRs. Pflugers Arch - Eur J Physiol 473, 1775–1793 (2021). https://doi.org/10.1007/s00424-021-02614-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02614-2