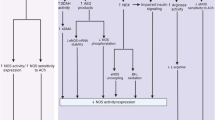
Abstract
Hyperuricemia, defined as elevated serum concentrations of uric acid (UA) above 416 µmol L−1, is related to the development of cardiometabolic disorders, probably via induction of endothelial dysfunction. Hyperuricemia causes endothelial dysfunction via induction of cell apoptosis, oxidative stress, and inflammation; however, it's interfering with insulin signaling and decreased endothelial nitric oxide (NO) availability, resulting in the development of endothelial insulin resistance, which seems to be a major underlying mechanism for hyperuricemia-induced endothelial dysfunction. Here, we elaborate on how hyperuricemia induces endothelial insulin resistance through the disruption of insulin-stimulated endothelial NO synthesis. High UA concentrations decrease insulin-induced NO synthesis within the endothelial cells by interfering with insulin signaling at either the receptor or post-receptor levels (i.e., proximal and distal steps). At the proximal post-receptor level, UA impairs the function of the insulin receptor substrate (IRS) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) in the insulin signaling pathway. At the distal level, high UA concentrations impair endothelial NO synthase (eNOS)-NO system by decreasing eNOS expression and activity as well as by direct inactivation of NO. Clinically, UA-induced endothelial insulin resistance is translated into impaired endothelial function, impaired NO-dependent vasodilation, and the development of systemic insulin resistance. UA-lowering drugs may improve endothelial function in subjects with hyperuricemia.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- ABC:
-
ATP-binding cassette transporter
- Ang-II:
-
Angiotensin II
- AP-1:
-
Activator protein-1
- BCRP:
-
Breast cancer resistance protein
- CI:
-
Confidence interval
- eNOS:
-
Endothelial nitric oxide synthase
- ENPP1:
-
Ectonucleotide pyrophosphatase/phosphodiesterase 1
- ERK:
-
Extracellular signal-regulated kinase
- ET-1:
-
Endothelin-1
- GLUT:
-
Glucose transporter
- HPX:
-
Hypoxanthine
- HUVEC:
-
Human umbilical vein endothelial cell
- IRS:
-
Insulin receptor substrate
- JNK:
-
C-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MCT:
-
Monocarboxylate transporter
- MRP:
-
Multidrug resistance-associated protein
- NF-κB:
-
Nuclear factor kappa-B
- NO:
-
Nitric oxide
- OAT:
-
Organic anion transporter
- PI3K:
-
Phosphatidylinositol 3-kinase
- PKC:
-
Protein kinase C
- ROS:
-
Reactive oxygen species
- RR:
-
Relative risk
- SLC:
-
Solute carrier family
- T2DM:
-
Type 2 diabetes mellitus
- UA:
-
Uric acid
- UAT:
-
Uric acid transporter
- URATv1:
-
Voltage-driven urate transporter 1
- VCAM-1:
-
Vascular cell adhesion molecule-1
- XDH:
-
Xanthine dehydrogenase
- XO:
-
Xanthine oxidase
- XOR:
-
Xanthine oxidoreductase
References
Abate N, Chandalia M, Di Paola R, Foster DW, Grundy SM, Trischitta V (2006) Mechanisms of disease: ectonucleotide pyrophosphatase phosphodiesterase 1 as a ‘gatekeeper’ of insulin receptors. Nat Clin Pract Endocrinol Metab 2:694–701. https://doi.org/10.1038/ncpendmet0367
Adachi SI, Yoshizawa F, Yagasaki K (2017) Hyperuricemia in type 2 diabetic model KK-A(y)/Ta mice: a potent animal model with positive correlation between insulin resistance and plasma high uric acid levels. BMC Res Notes 10:577. https://doi.org/10.1186/s13104-017-2897-x
Agarwal A, Banerjee A, Banerjee UC (2011) Xanthine oxidoreductase: a journey from purine metabolism to cardiovascular excitation-contraction coupling. Crit Rev Biotechnol 31:264–280. https://doi.org/10.3109/07388551.2010.527823
Amaya Y, Yamazaki K, Sato M, Noda K, Nishino T, Nishino T (1990) Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem 265:14170–14175
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 78:6858–6862. https://doi.org/10.1073/pnas.78.11.6858
Andreozzi F, Laratta E, Procopio C, Hribal ML, Sciacqua A, Perticone M, Miele C, Perticone F, Sesti G (2007) Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol Cell Biol 27:2372–2383. https://doi.org/10.1128/mcb.01340-06
Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G (2004) Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res 94:1211–1218. https://doi.org/10.1161/01.res.0000126501.34994.96
Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA (2001) Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98:15215–15220. https://doi.org/10.1073/pnas.221292098
Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, Johnson RJ, Sautin YY (2011) Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60:1258–1269
Barrett EJ, Liu Z (2013) The endothelial cell: an “early responder” in the development of insulin resistance. Rev Endocr Metab Disord 14:21–27. https://doi.org/10.1007/s11154-012-9232-6
Battelli MG, Bolognesi A, Polito L (2014) Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochem Biophys Acta 1842:1502–1517. https://doi.org/10.1016/j.bbadis.2014.05.022
Battelli MG, Polito L, Bortolotti M, Bolognesi A (2016) Xanthine oxidoreductase in drug metabolism: beyond a role as a detoxifying enzyme. Curr Med Chem 23:4027–4036. https://doi.org/10.2174/0929867323666160725091915
Becker BF (1993) Towards the physiological function of uric acid. Free Radical Biol Med 14:615–631. https://doi.org/10.1016/0891-5849(93)90143-I
Benn CL, Dua P, Gurrell R, Loudon P, Pike A, Storer RI, Vangjeli C (2018) Physiology of hyperuricemia and urate-lowering treatments. Front Med 5:160. https://doi.org/10.3389/fmed.2018.00160
Bobulescu IA, Moe OW (2012) Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis 19:358–371. https://doi.org/10.1053/j.ackd.2012.07.009
Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM (2006) Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke 37:1503–1507. https://doi.org/10.1161/01.STR.0000221716.55088.d4
Cai W, Duan X-M, Liu Y, Yu J, Tang Y-L, Liu Z-L, Jiang S, Zhang C-P, Liu J-Y, Xu J-X (2017) Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res Int 2017:4391920–4391920. https://doi.org/10.1155/2017/4391920
Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO (2010) Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA 107:17716–17720. https://doi.org/10.1073/pnas.1008872107
Cersosimo E, DeFronzo RA (2006) Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev 22:423–436. https://doi.org/10.1002/dmrr.634
Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, Lee BH (2014) Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J Off Publ Fed Am Soc Exp Biol 28:3197–3204. https://doi.org/10.1096/fj.13-247148
De Becker B, Coremans C, Chaumont M, Delporte C, Van Antwerpen P, Franck T, Rousseau A, Zouaoui Boudjeltia K, Cullus P, van de Borne P (2019) Severe hypouricemia impairs endothelium-dependent vasodilatation and reduces blood pressure in healthy young men: a randomized, placebo-controlled, and crossover study. J Am Heart Assoc 8:e013130. https://doi.org/10.1161/jaha.119.013130
De Nigris V, Pujadas G, La Sala L, Testa R, Genovese S, Ceriello A (2015) Short-term high glucose exposure impairs insulin signaling in endothelial cells. Cardiovasc Diabetol 14:114. https://doi.org/10.1186/s12933-015-0278-0
Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, Punzi L, Borghi C (2014) Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci 18:1295–1306
Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, Kearney MT (2008) Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes 57:3307–3314. https://doi.org/10.2337/db07-1111
Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U (2001) Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104:342–345
El Ridi R, Tallima H (2017) Physiological functions and pathogenic potential of uric acid: a review. J Adv Res 8:487–493. https://doi.org/10.1016/j.jare.2017.03.003
Fang J, Alderman MH (2000) Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 283:2404–2410. https://doi.org/10.1001/jama.283.18.2404
Fisslthaler B, Benzing T, Busse R, Fleming I (2003) Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: a dual role for Akt and AP-1. Nitric Oxide 8:253–261. https://doi.org/10.1016/s1089-8603(03)00042-9
George J, Carr E, Davies J, Belch JJ, Struthers A (2006) High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114:2508–2516. https://doi.org/10.1161/circulationaha.106.651117
Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN (2008) Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 27:967–978. https://doi.org/10.1080/15257770802257952
Ghasemi A (2021) Uric acid-induced pancreatic β-cell dysfunction. BMC Endocr Disord 21:24. https://doi.org/10.1186/s12902-021-00698-6
Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R (2000) Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275:7757–7763. https://doi.org/10.1074/jbc.275.11.7757
Grayson PC, Kim SY, LaValley M, Choi HK (2011) Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res 63:102–110. https://doi.org/10.1002/acr.20344
Hao L, Zhou Y, Xu H (2019) A study of the correlation between vitamin D uric acid levels and senile acute cerebral infarction. Int J Clin Exp Med 12:9343–9350
Hermann C, Assmus B, Urbich C, Zeiher AM, Dimmeler S (2000) Insulin-mediated stimulation of protein kinase Akt: a potent survival signaling cascade for endothelial cells. Arterioscler Thromb Vasc Biol 20:402–409. https://doi.org/10.1161/01.atv.20.2.402
Hille R, Nishino T (1995) Xanthine oxidase and xanthine dehydrogenase. FASEB J 9:995–1003. https://doi.org/10.1096/fasebj.9.11.7649415
Hong Q, Qi K, Feng Z, Huang Z, Cui S, Wang L, Fu B, Ding R, Yang J, Chen X, Wu D (2012) Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium 51:402–410. https://doi.org/10.1016/j.ceca.2012.01.003
Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA (1999) Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem 274:4985–4994. https://doi.org/10.1074/jbc.274.8.4985
Ichimori K, Fukahori M, Nakazawa H, Okamoto K, Nishino T (1999) Inhibition of xanthine oxidase and xanthine dehydrogenase by nitric oxide. Nitric oxide converts reduced xanthine-oxidizing enzymes into the desulfo-type inactive form. J Biol Chem 274:7763–7768. https://doi.org/10.1074/jbc.274.12.7763
Jakše B, Jakše B, Pajek M, Pajek J (2019) Uric acid and plant-based nutrition. Nutrients 11:1736. https://doi.org/10.3390/nu11081736
Johnson RJ, Gaucher EA, Sautin YY, Henderson GN, Angerhofer AJ, Benner SA (2008) The planetary biology of ascorbate and uric acid and their relationship with the epidemic of obesity and cardiovascular disease. Med Hypotheses 71:22–31. https://doi.org/10.1016/j.mehy.2008.01.017
Johnson RJ, Sautin YY, Oliver WJ, Roncal C, Mu W, Gabriela Sanchez-Lozada L, Rodriguez-Iturbe B, Nakagawa T, Benner SA (2009) Lessons from comparative physiology: could uric acid represent a physiologic alarm signal gone awry in western society? J Comp Physiol [B] 179:67–76. https://doi.org/10.1007/s00360-008-0291-7
Kang DH, Park SK, Lee IK, Johnson RJ (2005) Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16:3553–3562. https://doi.org/10.1681/asn.2005050572
Kato M, Hisatome I, Tomikura Y, Kotani K, Kinugawa T, Ogino K, Ishida K, Igawa O, Shigemasa C, Somers VK (2005) Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol 96:1576–1578. https://doi.org/10.1016/j.amjcard.2005.07.068
Kelley EE, Hock T, Khoo NK, Richardson GR, Johnson KK, Powell PC, Giles GI, Agarwal A, Lancaster JR Jr, Tarpey MM (2006) Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Radical Biol Med 40:952–959. https://doi.org/10.1016/j.freeradbiomed.2005.11.008
Kellogg EW 3rd, Fridovich I (1977) Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem 252:6721–6728
Kim J-a, Montagnani M, Koh KK, Quon MJ (2006) Reciprocal relationships between insulin resistance and endothelial dysfunction. Circulation 113:1888–1904. https://doi.org/10.1161/CIRCULATIONAHA.105.563213
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA (2010) Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res 62:170–180. https://doi.org/10.1002/acr.20065
Ko J, Kang HJ, Kim DA, Kim MJ, Ryu ES, Lee S, Ryu JH, Roncal C, Johnson RJ, Kang DH (2019) Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J Off Publ Fed Am Soc Exp Biol 33:13334–13345. https://doi.org/10.1096/fj.201901148R
Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, Saito A, Sone H (2009) Association between serum uric acid and development of type 2 diabetes. Diabetes Care 32:1737–1742. https://doi.org/10.2337/dc09-0288
Komori H, Yamada K, Tamai I (2018) Hyperuricemia enhances intracellular urate accumulation via down-regulation of cell-surface BCRP/ABCG2 expression in vascular endothelial cells. Biochim Biophys Acta 1860:973–980. https://doi.org/10.1016/j.bbamem.2018.01.006
Konta T, Ichikawa K, Kawasaki R, Fujimoto S, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Narita I, Kondo M, Shibagaki Y, Kasahara M, Asahi K, Watanabe T (2020) Association between serum uric acid levels and mortality: a nationwide community-based cohort study. Sci Rep 10:6066. https://doi.org/10.1038/s41598-020-63134-0
Kuzkaya N, Weissmann N, Harrison DG, Dikalov S (2005) Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 70:343–354. https://doi.org/10.1016/j.bcp.2005.05.009
Lai J-H, Luo S-F, Hung L-F, Huang C-Y, Lien S-B, Lin L-C, Liu F-C, Yen BL, Ho L-J (2017) Physiological concentrations of soluble uric acid are chondroprotective and anti-inflammatory. Sci Rep 7:2359. https://doi.org/10.1038/s41598-017-02640-0
Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P (2014) Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 15:122–122. https://doi.org/10.1186/1471-2369-15-122
Li P, Zhang L, Zhang M, Zhou C, Lin N (2016) Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: a mechanism for uric acid-induced endothelial dysfunction. Int J Mol Med 37:989–997. https://doi.org/10.3892/ijmm.2016.2491
Liang WY, Zhu XY, Zhang JW, Feng XR, Wang YC, Liu ML (2015) Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-kappa B signaling. Nutr Metab Cardiovasc Dis 25:187–194. https://doi.org/10.1016/j.numecd.2014.08.006
Liu S, Yuan Y, Zhou Y, Zhao M, Chen Y, Cheng J, Lu Y, Liu J (2017) Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and GLUT9-mediated uric acid uptake. J Cell Mol Med 21:2553–2562. https://doi.org/10.1111/jcmm.13176
Lu J, He Y, Cui L, Xing X, Liu Z, Li X, Zhang H, Li H, Sun W, Ji A, Wang Y, Yin H, Li C (2020) Hyperuricemia pedisposes to the onset of diabetes via promoting pancreatic β-cell death in uricase-deficient male mice. Diabetes 69:1149–1163. https://doi.org/10.2337/db19-0704
Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, Huang K, Zhang C (2013) High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS ONE 8:e56864. https://doi.org/10.1371/journal.pone.0056864
Madonna R, Pandolfi A, Massaro M, Consoli A, De Caterina R (2004) Insulin enhances vascular cell adhesion molecule-1 expression in human cultured endothelial cells through a pro-atherogenic pathway mediated by p38 mitogen-activated protein-kinase. Diabetologia 47:532–536. https://doi.org/10.1007/s00125-004-1330-x
Maeno Y, Li Q, Park K, Rask-Madsen C, Gao B, Matsumoto M, Liu Y, Wu IH, White MF, Feener EP, King GL (2012) Inhibition of insulin signaling in endothelial cells by protein kinase C-induced phosphorylation of p85 subunit of phosphatidylinositol 3-kinase (PI3K). J Biol Chem 287:4518–4530. https://doi.org/10.1074/jbc.M111.286591
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Regulation of uric acid metabolism and excretion. Int J Cardiol 213:8–14. https://doi.org/10.1016/j.ijcard.2015.08.109
Martillo MA, Nazzal L, Crittenden DB (2014) The crystallization of monosodium urate. Curr Rheumatol Rep 16:400–400. https://doi.org/10.1007/s11926-013-0400-9
Martínez-Sánchez FD, Vargas-Abonce VP, Guerrero-Castillo AP, Santos-Villavicencio ML, Eseiza-Acevedo J, Meza-Arana CE, Gulias-Herrero A, Gómez-Sámano M (2020) Serum Uric Acid concentration is associated with insulin resistance and impaired insulin secretion in adults at risk for Type 2 Diabetes. Prim Care Diabetes. https://doi.org/10.1016/j.pcd.2020.10.006
Maruhashi T, Hisatome I, Kihara Y, Higashi Y (2018) Hyperuricemia and endothelial function: from molecular background to clinical perspectives. Atherosclerosis 278:226–231. https://doi.org/10.1016/j.atherosclerosis.2018.10.007
Mishima M, Hamada T, Maharani N, Ikeda N, Onohara T, Notsu T, Ninomiya H, Miyazaki S, Mizuta E, Sugihara S (2016) Effects of uric acid on the NO production of HUVECs and its restoration by urate lowering agents. Drug Research 66:270–274
Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B (2002) Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277:1794–1799. https://doi.org/10.1074/jbc.M103728200
Muniyappa R, Chen H, Montagnani M, Sherman A, Quon MJ (2020) Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am J Phys Endocrinol Metab 319:E629–E646. https://doi.org/10.1152/ajpendo.00247.2020
Muniyappa R, Iantorno M, Quon MJ (2008) An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am 37:685–x. https://doi.org/10.1016/j.ecl.2008.06.001
Muraoka S, Miura T (2003) Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol 93:284–289. https://doi.org/10.1111/j.1600-0773.2003.pto930606.x
Ndrepepa G (2018) Uric acid and cardiovascular disease. Clin Chim Acta Int J Clin Chem 484:150–163. https://doi.org/10.1016/j.cca.2018.05.046
Oliver FJ, De la Rubia G, Feener E, Lee M, Loeken M, Shiba T, Quertermous T, King G (1991) Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem 266:23251–23256
Osses N, Pearson JD, Yudilevich DL, Jarvis SM (1996) Hypoxanthine enters human vascular endothelial cells (ECV 304) via the nitrobenzylthioinosine-insensitive equilibrative nucleoside transporter. Biochem J 317(Pt 3):843–848. https://doi.org/10.1042/bj3170843
Otani N, Toyoda S, Sakuma M, Hayashi K, Ouchi M, Fujita T, Anzai N, Tanaka A, Node K, Uemura N, Inoue T (2018) Effects of uric acid on vascular endothelial function from bedside to bench. Hypertens Res 41:923–931. https://doi.org/10.1038/s41440-018-0095-4
Panus PC, Wright SA, Chumley PH, Radi R, Freeman BA (1992) The contribution of vascular endothelial xanthine dehydrogenase/oxidase to oxygen-mediated cell injury. Arch Biochem Biophys 294:695–702. https://doi.org/10.1016/0003-9861(92)90743-g
Park J-H, Jin YM, Hwang S, Cho D-H, Kang D-H, Jo I (2013) Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide 32:36–42. https://doi.org/10.1016/j.niox.2013.04.003
Partridge CA, Blumenstock FA, Malik AB (1992) Pulmonary microvascular endothelial cells constitutively release xanthine oxidase. Arch Biochem Biophys 294:184–187. https://doi.org/10.1016/0003-9861(92)90155-P
Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M (2005) Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289:H813-822. https://doi.org/10.1152/ajpheart.00092.2005
Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, Endou H, Johnson RJ (2006) Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol 17:1791–1795. https://doi.org/10.1681/asn.2006030264
Radi R, Tan S, Prodanov E, Evans RA, Parks DA (1992) Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta Protein Struct Mol Enzymol 1122:178–182. https://doi.org/10.1016/0167-4838(92)90321-4
Rask-Madsen C, King GL (2013) Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17:20–33. https://doi.org/10.1016/j.cmet.2012.11.012
Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL, (2010) Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11:379–389. https://doi.org/10.1016/j.cmet.2010.03.013
Rouquette M, Page S, Bryant R, Benboubetra M, Stevens CR, Blake DR, Whish WD, Harrison R, Tosh D (1998) Xanthine oxidoreductase is asymmetrically localised on the outer surface of human endothelial and epithelial cells in culture. FEBS Lett 426:397–401. https://doi.org/10.1016/s0014-5793(98)00385-8
Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ (2012) Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol 121:e71–e78. https://doi.org/10.1159/000345509
Sánchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodríguez-Iturbe B, Johnson RJ (2008) Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol 295:F1134-1141. https://doi.org/10.1152/ajprenal.00104.2008
Sánchez-Lozada LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, Nepomuceno T, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J (2005) Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 67:237–247. https://doi.org/10.1111/j.1523-1755.2005.00074.x
Schlotte V, Sevanian A, Hochstein P, Weithmann KU (1998) Effect of uric acid and chemical analogues on oxidation of human low density lipoprotein in vitro. Free Radical Biol Med 25:839–847. https://doi.org/10.1016/s0891-5849(98)00160-9
Schulman IH, Zhou MS (2009) Vascular insulin resistance: a potential link between cardiovascular and metabolic diseases. Curr Hypertens Rep 11:48–55. https://doi.org/10.1007/s11906-009-0010-0
Schwartz IF, Grupper A, Chernichovski T, Grupper A, Hillel O, Engel A, Schwartz D (2011) Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res 48:252–260. https://doi.org/10.1159/000320356
So A, Thorens B (2010) Uric acid transport and disease. J Clin Investig 120:1791–1799. https://doi.org/10.1172/jci42344
Sorensen LB (1965) Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum Off J Am Coll Rheumatol 8:694–706
Sugihara S, Hisatome I, Kuwabara M, Niwa K, Maharani N, Kato M, Ogino K, Hamada T, Ninomiya H, Higashi Y, Ichida K, Yamamoto K (2015) Depletion of uric acid due to SLC22A12 (URAT1) loss-of-function mutation causes endothelial dysfunction in hypouricemia. Circ J 79:1125–1132. https://doi.org/10.1253/circj.CJ-14-1267
Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM (2013) Protein kinase C-β contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation 127:86–95. https://doi.org/10.1161/circulationaha.112.127514
Tan S, Radi R, Gaudier F, Evans RA, Rivera A, Kirk KA, Parks DA (1993) Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr Res 34:303–307. https://doi.org/10.1203/00006450-199309000-00013
Tanigaki K, Mineo C, Yuhanna IS, Chambliss KL, Quon MJ, Bonvini E, Shaul PW (2009) C-reactive protein inhibits insulin activation of endothelial nitric oxide synthase via the immunoreceptor tyrosine-based inhibition motif of FcgammaRIIB and SHIP-1. Circ Res 104:1275–1282. https://doi.org/10.1161/circresaha.108.192906
Tassone EJ, Cimellaro A, Perticone M, Hribal ML, Sciacqua A, Andreozzi F, Sesti G, Perticone F (2018) Uric acid impairs insulin signaling by promoting Enpp1 binding to insulin receptor in human umbilical vein endothelial cells. Front Endocrinol (Lausanne) 9:98–98. https://doi.org/10.3389/fendo.2018.00098
Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR (2003) The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Investig 111:1373–1380. https://doi.org/10.1172/JCI15211
Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, Li J (2014) Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS ONE 9:e114259. https://doi.org/10.1371/journal.pone.0114259
Wang R, Song Y, Yan Y, Ding Z (2016) Elevated serum uric acid and risk of cardiovascular or all-cause mortality in people with suspected or definite coronary artery disease: a meta-analysis. Atherosclerosis 254:193–199. https://doi.org/10.1016/j.atherosclerosis.2016.10.006
Waring WS, McKnight JA, Webb DJ, Maxwell SRJ (2006) Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 55:3127–3132. https://doi.org/10.2337/db06-0283
Williams AW, Wilson DM (1990) Uric acid metabolism in humans. Semin Nephrol 10:9–14
Wright AF, Rudan I, Hastie ND, Campbell H (2010) A ‘complexity’ of urate transporters. Kidney Int 78:446–452. https://doi.org/10.1038/ki.2010.206
Xie H, Sun J, Chen Y, Zong M, Li S, Wang Y (2015) EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid Med Cell Longev 2015:214836–214836. https://doi.org/10.1155/2015/214836
Xu L, Shi Y, Zhuang S, Liu N (2017) Recent advances on uric acid transporters. Oncotarget 8:100852–100862. https://doi.org/10.18632/oncotarget.20135
Yang B, Li S, Zhu J, Huang S, Zhang A, Jia Z, Ding G, Zhang Y (2020) MiR-214 protects against uric acid-induced endothelial cell apoptosis. Front Med 7:411
Yang X, Gu J, Lv H, Li H, Cheng Y, Liu Y, Jiang Y (2019) Uric acid induced inflammatory responses in endothelial cells via upregulating (pro)renin receptor. Biomed Pharmacother 109:1163–1170. https://doi.org/10.1016/j.biopha.2018.10.129
Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH (2010) Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens 28:1234–1242
Yuan H, Hu Y, Zhu Y, Zhang Y, Luo C, Li Z, Wen T, Zhuang W, Zou J, Hong L, Zhang X, Hisatome I, Yamamoto T, Cheng J (2017) Metformin ameliorates high uric acid-induced insulin resistance in skeletal muscle cells. Mol Cell Endocrinol 443:138–145. https://doi.org/10.1016/j.mce.2016.12.025
Yuan H, Yu C, Li X, Sun L, Zhu X, Zhao C, Zhang Z, Yang Z (2015) Serum uric acid levels and risk of metabolic syndrome: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab 100:4198–4207
Zhang Y, Zhan RX, Chen JQ, Gao Y, Chen L, Kong Y, Zhong XJ, Liu MQ, Chu JJ, Yan GQ, Li T, He M, Huang QR (2015) Pharmacological activation of PPAR gamma ameliorates vascular endothelial insulin resistance via a non-canonical PPAR gamma-dependent nuclear factor-kappa B trans-repression pathway. Eur J Pharmacol 754:41–51. https://doi.org/10.1016/j.ejphar.2015.02.004
Zhao L, Cao L, Zhao TY, Yang X, Zhu XX, Zou HJ, Wan WG, Xue Y (2020) Cardiovascular events in hyperuricemia population and a cardiovascular benefit-risk assessment of urate-lowering therapies: a systematic review and meta-analysis. Chin Med J 133:982–993. https://doi.org/10.1097/cm9.0000000000000682
Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J (2008) Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 295:C1183-1190. https://doi.org/10.1152/ajpcell.00075.2008
Zhen H, Gui F (2017) The role of hyperuricemia on vascular endothelium dysfunction. Biomed Rep 7:325–330. https://doi.org/10.3892/br.2017.966
Zhi L, Yuzhang Z, Tianliang H, Hisatome I, Yamamoto T, Jidong C (2016) High uric acid induces insulin resistance in cardiomyocytes in vitro and in vivo. PLoS ONE 11:e0147737. https://doi.org/10.1371/journal.pone.0147737
Zhu Y, Hu Y, Huang T, Zhang Y, Li Z, Luo C, Luo Y, Yuan H, Hisatome I, Yamamoto T, Cheng J (2014) High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem Biophys Res Commun 447:707–714. https://doi.org/10.1016/j.bbrc.2014.04.080
Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F (2006) Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 17:1466–1471. https://doi.org/10.1681/asn.2005090949
Zuo T, Liu X, Jiang L, Mao S, Yin X, Guo L (2016) Hyperuricemia and coronary heart disease mortality: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord 16:207. https://doi.org/10.1186/s12872-016-0379-z
Zweier JL, Kuppusamy P, Lutty GA (1988) Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci 85:4046–4050
Funding
This study has been supported by Shahid Beheshti University of Medical Sciences (Grant Number 25401), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Idea and conceptualization: Asghar Ghasemi and Zahra Bahadoran
Writing, reviewing, and editing: Zahra Bahadoran, Asghar Ghasemi, Khosrow Kashfi, Parvin Mirmiran
Literature research: Zahra Bahadoran, Asghar Ghasemi, Khosrow Kashfi, Parvin Mirmiran
Figure conceptualization and design: Zahra Bahadoran and Asghar Ghasemi
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on pathophysiological mechanisms of cardiometabolic diseases in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Bahadoran, Z., Mirmiran, P., Kashfi, K. et al. Hyperuricemia-induced endothelial insulin resistance: the nitric oxide connection. Pflugers Arch - Eur J Physiol 474, 83–98 (2022). https://doi.org/10.1007/s00424-021-02606-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02606-2