Abstract
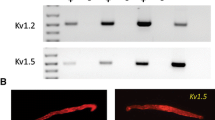
The group of KCNQ-encoded voltage-gated potassium (Kv7) channels includes five family members (Kv7.1–7.5). We examined the molecular expression and functional roles of Kv7 channels in corporal smooth muscle (CSM). Isolated rabbit CSM strips were mounted in an organ bath system to characterize Kv7 channels during CSM relaxation. Intracellular Ca2+ levels were measured in the CSM using the Ca2+ dye Fluo-4 AM. The expression of the KCNQ1–5 (the encoding genes for Kv7.1–7.5) and KCNE1–5 subtypes was determined by quantitative real-time PCR. Electrophysiological recordings and an in situ proximity ligation assay (PLA) were also performed. ML213 (a Kv7.2/7.4/7.5 activator) exhibited the most potent relaxation effect. XE911 (a Kv7.1–7.5 blocker) significantly inhibited the relaxation caused by ML213. Removal of the endothelium from the CSM did not affect the relaxation effect of ML213. H-89 (a protein kinase A inhibitor) and ESI-09 (an exchange protein directly activated by cAMP inhibitor) significantly inhibited ML213-induced relaxation (H-89: 31.3%; ESI-09: 52.7%). XE991 significantly increased basal [Ca2+]i in hCSM cells. KCNQ4 (the Kv7.4-encoding gene) and KCNE4 in CSM were the most abundantly expressed subtypes in humans and rats, respectively. KCNQ4 and KCNE4 expression was significantly decreased in diabetes mellitus rats. ML213 significantly increased the outward current amplitude. XE991 inhibited the ML213-induced outward currents. ML213 hyperpolarized the hCSM cell membrane potential. Subsequent addition of XE991 completely reversed the ML213-induced hyperpolarizing effects. A combination of Kv7.4 and Kv7.5 antibodies generated a strong PLA signal. We found that the Kv7.4 channel is a potential target for ED treatment.










Similar content being viewed by others
References
(1993) NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 270:83–90
(2010-) Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US), Bethesda (MD)
Abbott GW, Goldstein SA (2001) Potassium channel subunits encoded by the KCNE gene family: physiology and pathophysiology of the MinK-related peptides (MiRPs). Mol Interv 1:95–107
Amato G, Roeloffs R, Rigdon GC, Antonio B, Mersch T, McNaughton-Smith G, Wickenden AD, Fritch P, Suto MJ (2011) N-Pyridyl and pyrimidine benzamides as KCNQ2/Q3 potassium channel openers for the treatment of epilepsy. ACS Med Chem Lett 2:481–484
Brueggemann LI, Haick JM, Cribbs LL, Byron KL (2014) Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol 86:330–341
Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, Byron KL (2014) Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem 289:2099–2111
Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA (2014) Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34:887–893
Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA (2012) Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension 59:877–884
Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB (1994) Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol 151:54–61
Fosmo AL, Skraastad OB (2017) The Kv7 channel and cardiovascular risk factors. Front Cardiovasc Med 4:75
Haick JM, Byron KL (2016) Novel treatment strategies for smooth muscle disorders: targeting Kv7 potassium channels. Pharmacol Ther 165:14–25
Jacob S, Nair AB (2016) An updated overview on therapeutic drug monitoring of recent antiepileptic drugs. Drugs R D 16:303–316
Jepps TA, Carr G, Lundegaard PR, Olesen SP, Greenwood IA (2015) Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J Physiol 593:5325–5340
Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA (2011) Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 124:602–611
Jepps TA, Olesen SP, Greenwood IA, Dalsgaard T (2016) Molecular and functional characterization of Kv 7 channels in penile arteries and corpus cavernosum of healthy and metabolic syndrome rats. Br J Pharmacol 173:1478–1490
Mani BK, Robakowski C, Brueggemann LI, Cribbs LL, Tripathi A, Majetschak M, Byron KL (2016) Kv7.5 potassium channel subunits are the primary targets for PKA-dependent enhancement of vascular smooth muscle Kv7 currents. Mol Pharmacol 89:323–334
Mattmann ME, Yu H, Lin Z, Xu K, Huang X, Long S, Wu M, McManus OB, Engers DW, Le UM, Li M, Lindsley CW, Hopkins CR (2012) Identification of (R)-N-(4-(4-methoxyphenyl)thiazol-2-yl)-1-tosylpiperidine-2-carboxamide, ML277, as a novel, potent and selective K(v)7.1 (KCNQ1) potassium channel activator. Bioorg Med Chem Lett 22:5936–5941
Miceli F, Soldovieri MV, Martire M, Taglialatela M (2008) Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr Opin Pharmacol 8:65–74
Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA (2011) Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162:42–53
Palmer LS, Valcic M, Melman A, Giraldi A, Wagner G, Christ GJ (1994) Characterization of cyclic AMP accumulation in cultured human corpus cavernosum smooth muscle cells. J Urol 152:1308–1314
Park HJ, Moon KH, Lee SW, Lee WK, Kam SC, Lee JH, Park NC (2014) Mirodenafil for the treatment of erectile dysfunction: a systematic review of the literature. World J Mens Health 32:18–27
Rehman J, Chenven E, Brink P, Peterson B, Walcott B, Wen YP, Melman A, Christ G (1997) Diminished neurogenic but not pharmacological erections in the 2- to 3-month experimentally diabetic F-344 rat. Am J Phys Heart Circ Phys 272:H1960–H1971
Rundfeldt C, Netzer R (2000) Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung 50:1063–1070
Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ (2000) A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403:196–199
Stott JB, Barrese V, Greenwood IA (2016) Kv7 channel activation underpins EPAC-dependent relaxations of rat arteries. Arterioscler Thromb Vasc Biol 36:2404–2411
Strutz-Seebohm N, Seebohm G, Fedorenko O, Baltaev R, Engel J, Knirsch M, Lang F (2006) Functional coassembly of KCNQ4 with KCNE-beta- subunits in Xenopus oocytes. Cell Physiol Biochem 18:57–66
Sung HH, Chae MR, So I, Jeon JH, Park JK, Lee SW (2011) Effects of ginsenoside on large-conductance K (Ca) channels in human corporal smooth muscle cells. Int J Impot Res 23:193–199
Sung HH, Kang SJ, Chae MR, Kim HK, Park JK, Kim CY, Lee SW (2017) Effect of BKCa channel opener LDD175 on erectile function in an in vivo diabetic rat model. J Sex Med 14:59–68
Supuran CT, Mastrolorenzo A, Barbaro G, Scozzafava A (2006) Phosphodiesterase 5 inhibitors--drug design and differentiation based on selectivity, pharmacokinetic and efficacy profiles. Curr Pharm Des 12:3459–3465
Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D (1998) KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282:1890–1893
Wickenden AD, Krajewski JL, London B, Wagoner PK, Wilson WA, Clark S, Roeloffs R, McNaughton-Smith G, Rigdon GC (2008) N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol 73:977–986
Wrobel E, Tapken D, Seebohm G (2012) The KCNE tango - how KCNE1 interacts with Kv7.1. Front Pharmacol 3:142
Yeung SY, Pucovsky V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA (2007) Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol 151:758–770
Yu H, Wu M, Townsend SD, Zou B, Long S, Daniels JS, McManus OB, Li M, Lindsley CW, Hopkins CR (2011) Discovery, synthesis, and structure activity relationship of a series of N-aryl- bicyclo[2.2.1]heptane-2-carboxamides: characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem Neurosci 2:572–577
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C0982).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.H., Chae, M.R., Kang, S.J. et al. Characterization and functional roles of KCNQ-encoded voltage-gated potassium (Kv7) channels in human corpus cavernosum smooth muscle. Pflugers Arch - Eur J Physiol 472, 89–102 (2020). https://doi.org/10.1007/s00424-019-02343-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02343-7