Abstract
The abilities to detect warmth and heat are critical for the survival of all animals, both in order to be able to identify suitable thermal environments for the many different activities essential for life and to avoid damage caused by extremes of temperature. Several ion channels belonging to the TRP family are activated by non-noxious warmth or by heat and are therefore plausible candidates for thermal detectors, but identifying those that actually regulate warmth and heat detection in intact animals has proven problematic. TRPM2 has recently emerged as a likely candidate for the detector of non-noxious warmth, as it is expressed in sensory neurons, and mice show deficits in the detection of warmth when TRPM2 is genetically deleted. TRPM2 is a chanzyme, containing a thermally activated TRP ion channel domain attached to a C-terminal motif, derived from a mitochondrial ADP ribose pyrophosphatase, that confers on the channel sensitivity to ADP ribose and reactive oxygen species such as hydrogen peroxide. Several open questions remain. Male mammals prefer cooler environments than female, but the molecular basis of this sex difference is unknown. TRPM2 plays a role in regulating body temperature, but are other warmth-detecting mechanisms also involved? TRPM2 is expressed in autonomic neurons, but does it confer a sensory function in addition to the well-known motor functions of autonomic neurons? TRPM2 is thought to play important roles in the immune system, in pain and in insulin secretion, but the mechanisms are unclear. TRPM2 has to date received less attention than many other members of the TRP family but is rapidly assuming importance both in normal physiology and as a key target in disease pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to survive, all animals must detect environmental temperatures and react appropriately to them. Extreme temperatures can cause damage, and even subtle changes in the environmental temperature can cause changes in the physiology of living organisms. Animals must therefore be capable of detecting noxious thermal extremes, to avoid injury from extremes of heat or cold, and must also be equipped with the ability to detect optimal environmental temperatures in which to carry out different activities such as hunting, feeding, resting, and reproducing.
Thermal stimuli can be broadly categorized into painful heat, innocuous warmth, innocuous coolness, and painful cold. The mean detection thresholds for innocuous warmth and innocuous coolness range between 1.3 to 6.2 °C above and 0.9 to 3.4 °C below the baseline skin temperature, respectively. The mean detection threshold for painful heat is between 41.5 and 47.0 °C and for painful cold is more variable, at between 7.3 and 18.4 °C [95]. Differences in age, in the anatomical region tested, in ethnicity, and in sex have all been shown to influence the thermal detection thresholds [20, 26, 66, 67, 95]. Generally, the older the age, the higher the thresholds for detection of thermal stimuli, while face and hands are usually more sensitive [67, 95]. Ethnicity differences in thermal detection and thermal pain between Chinese and Danes have been reported, with people of Chinese origin being more sensitive to thermal stimuli [132]. Sex differences in response to thermal stimuli are most prominent for thermal pain thresholds, with females being more sensitive to both painful heat and painful cold [95, 121]. Although most studies did not show a statistically significant difference between sexes in the thresholds for thermal detection of innocuous warmth or coolness, several studies have shown a sex difference in thermal preference. The preferred environmental temperature for human males, 22 °C, is significantly lower than that for females (25 °C) [5, 48]. Moreover, one study found that females are more likely to show thermal discomfort in cool environments while males to be more likely to feel uncomfortable in warm environments [128]. In addition, women have a higher mean core temperature and a lower mean skin temperature than men [52].
The cellular mechanisms of thermosensation were initially examined by recording the activation of afferent nerve fibers in response to thermal stimuli in the glossopharyngeal nerve and the chorda tympani of cats [135]. Studies with lingual nerves also showed that both cold and warm receptors existed [136], and that receptor activation elicited steady discharges of impulses at constant temperatures, with maximum frequencies observed between 25 and 35 °C for cold receptors and 37.5 and 40 °C for warm receptors [19, 37]. Discharges elicited by thermal stimuli were shown in later studies in afferents from the cat’s skin [35, 36, 44]. Based on the conduction velocity of the electrical discharges, the transmission of the thermal stimuli signal was found to rely on unmyelinated C fibers or thinly myelinated Aδ fibers [16, 35, 44]. The detection of skin temperature in subprimates and primates was also shown to depend on cutaneous thermoreceptors and the propagation of action potentials in slowly conducting nerve fibers [16, 35, 44].
TRP ion channels activated by warmth and heat
Although the existence of thermoreceptors was demonstrated more than 80 years ago in the studies mentioned above, the molecular mechanisms underlying the detection of skin temperature by thermoreceptors remained elusive until recently, when noxious heat stimuli were shown to rapidly elicit an inward current in a subpopulation of small primary sensory neurons [11]. The reversal potential of the heat-activated ion current was very close to 0 mV, showing that the heat-activated ion channel discriminates poorly among cations. One of the heat-activated ion channels responsible for the heat sensitivity of primary sensory neurons was subsequently cloned by adopting a strategy based on calcium imaging of HEK293 cells expressing cDNA isolated from sensory neurons, and using capsaicin, the “hot” ingredient of chili peppers, to isolate a functional cDNA encoding a capsaicin receptor [10]. Capsaicin is a member of the chemical family named the vanilloids, and the channel was at first called vanilloid receptor subtype 1 (VR1) [10], though to recognize its homology with other members of the transient receptor potential (TRP) ion channel family, it has now been renamed transient receptor potential vanilloid 1 (TRPV1) under the unified nomenclature for the family of TRP cation channels [15]. Several other TRP channels activated by warmth or heat were identified soon after the cloning of TRPV1. The definition of thermosensitivity is based on the temperature coefficient (Q10) value, which is calculated as the relative increase in current amplitude when the temperature increases by 10 °C. Heat-sensitive TRP channels are generally defined as TRP channels with Q10 values higher than 5, and the thermally sensitive TRP channels have been identified on this criterion as TRPV1, TRPV2, TRPV3, TRPV4, TRPM2, TRPM3, TRPM4, and TRPM5 [123].
TRPV1 has a Q10 value of 25, an activation threshold at temperature > 42 °C, and carries a nonselective cation current displaying outward rectification [122]. In addition to heat stimuli and capsaicin, a wide range of other agonists, including resiniferatoxin [97], ethanol [116], anandamide [99], and extracellular protons [113] also activate TRPV1. Both C and Aδ nociceptive fibers of somatosensory neurons express TRPV1, and many TRPV1-positive fibers also co-express the proinflammatory neuropeptides substance P, neurokinin A, and calcitonin gene-related peptide (CGRP), substantiating a proinflammatory role for TRPV1 [46, 56, 113]. Shortly after the cloning of TRPV1, the same group also successfully cloned a TRPV1 homolog, subsequently named TRPV2 [9]. TRPV2 has a Q10 value higher than 100, with an even higher activation threshold, at 52 °C, than TRPV1. Based on its thermal sensitivity, TRPV2 was initially proposed as a candidate for a high-temperature, TRPV1-independent mechanism of heat sensation [9]. Like TRPV1, the activation of TRPV2 elicits a nonselective cation current displaying outward rectification [15, 63]. In addition to heat, hypo-osmolarity, membrane stretch, and cannabinoids also activate TRPV2 [77, 93]. One notable difference from TRPV1 is that TRPV2 is expressed in medium- to large-diameter myelinated sensory neurons, and in addition to sensory neurons, TRPV2 is also expressed in the epithelium of several different tissues (including pancreatic duct, mammary gland, parotid gland, submandibular gland, renal tubule, and tracheal gland) and in immune cells [59, 65].
TRPV3, also a close homolog of TRPV1, has a Q10 value higher than 6 and an activation threshold at temperatures around 31 °C, and carries an outwardly rectifying nonselective cation current [15, 86, 100, 130]. Carvacrol, eugenol, and thymol, found in plants such as oregano, savory, clove, and thyme, are agonists for TRPV3 [129]. TRPV4 was cloned as an osmotically sensitive channel carrying an outwardly rectifying nonselective cation current [14, 64, 105] and was found to be thermally sensitive almost 2 years after cloning [29]. The activation threshold of TRPV4 by non-noxious heat is around 25 °C [14] with a Q10 value of around 10 [29]. In addition to hypotonic stress and non-noxious heat, bisandrographolide from the Chinese herbal plant Andrographis paniculata can also activate TRPV4 [101]. Both TRPV3 and TRPV4 are expressed in skin keratinocytes, but not in neurons, and have therefore been proposed to act as non-noxious warmth sensors whose excitation could be transferred to primary afferent terminals of sensory neurons by a factor released from keratinocytes [13, 68, 86].
TRPM2 was cloned in 1998 with an initial goal of finding genes responsible for several diseases such as autoimmune polyglandular disease type I, bipolar affective disorder, and nonsyndromic hereditary deafness [78]. TRPM2 carries a voltage-insensitive nonselective cation current with a linear current-voltage relation. The thermosensitivity of TRPM2 was not discovered until 2006, when it was demonstrated that TRPM2 can be activated upon exposure to a temperature above 35 °C, with a Q10 value at around 15.6 [112]. In addition to heat, the activity of TRPM2 can be synergistically modulated by intracellular ADP-ribose (ADPR) and Ca2+ [34] and can also be enhanced by additional factors, such as cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) [4, 58]. Reactive oxygen species (ROS), such as H2O2, can also cause activation or sensitization of TRPM2 [30, 49, 126]. TRPM3, another member of the TRPM subfamily, carries an outwardly rectifying nonselective cation current and has an activation threshold at a temperature above 40 °C, with a Q10 value of 7.2 [124]. Pregnenolone sulphate, nifedipine, and β-cyclodextrin have been shown to activate TRPM3 [79, 125]. TRPM3 is abundantly expressed in small diameter dorsal root ganglion neurons and also in a variety of other tissues, including kidney, liver, ovary, brain, spinal cord, pituitary, vascular smooth muscle, and testis [27, 62, 79, 124].
Unlike most of the other members of the TRPM subfamily, which are nonselective cationic channels, TRPM4 and TRPM5 are not permeable to divalent cations [28]. TRPM4 carries an outwardly rectifying current that has a Q10 value of around 8.5 between 15 and 25 °C [108]. Intracellular calcium, decavanadate and BTP2 are also activators of TRPM4, in addition to heat [80, 107, 120]. TRPM4 is widely expressed in a variety of tissues, with high expression in the heart, pancreas, placenta, and prostate, and lower levels in the kidney, skeletal muscle, liver, intestines, thymus, and spleen in humans [1]. TRPM5 carries an outwardly rectifying current that has a Q10 value of around 10.3 with a threshold between 15 and 25 °C. Like TRPM4, TRPM5 can also be activated directly by intracellular calcium, and the concentration of intracellular calcium leading to activation of TRPM5 is even lower than that of TRPM4, though higher concentrations of intracellular calcium cause inhibition of TRPM5 [92, 120].
Roles of TRP channels as physiological sensors of warmth and heat
Although all the heat-sensitive TRP channels mentioned above display Q10 values higher than 5 in vitro, and therefore are plausible thermal sensors covering the entire range over which thermal detection is known in take place in animals, attempts to identify the thermosensors that are actually responsible for the physiological detection of warmth and heat in intact animals have not in general met with great success. TRPV1 knockout mice show a partial deficit in sensing noxious heat, but they still withdraw their tails from hot water [8], and, more importantly, no significant difference was noted between TRPV1 knockout mice and wild-type mice when evaluated with the two-plate thermal preference test [91]. Although TRPV2 was at first suggested to be responsible for the TRPV1-independent mechanism of heat sensation, behavioral assays of TRPV1/TRPV2 double knockout mice, or TRPV2 knockout mice treated with resiniferatoxin to desensitize TRPV1-expressing afferents, reveal no thermosensory deficits resulting from genetic deletion of TRPV2 [83]. Genetic deletion of TRPV3 in mice on an intercrossed C57BL6/129J background was reported initially to cause relatively minor deficits in responses to innocuous and noxious heat stimuli [75]. However, TRPV3 knockout mice on a homogeneous C57BL6 background exhibited no obvious changes in thermal preference behavior, while deletion of TRPV3 in a 129S6 background resulted in a more restricted range of occupancy centered around cooler floor temperatures [43]. Meanwhile, TRPV3 knockout mice showed no deficits in acute heat nociception on either a C57BL6 or a 129S6 background [43]. Importantly, although the activation threshold for TRPV3 and TRPV4 by heat is in the range of innocuous warmth, mice deficient in both TRPV3 and TRPV4 show a thermal preference behavior on a thermal gradient similar to wild-type mice and little or no change in acute heat perception [43]. These results demonstrate that TRPV3 and TRPV4 do not play important roles in thermosensation and that their contribution to thermosensation, if any, can be strongly influenced by genetic background. Mice with a genetic deletion of TRPM3 exhibited reduced but not absent sensitivity to noxious heat, in a similar manner to TRPV1 knockout mice. Although during the exploration period TRPM3 knockout mice spend significantly more time at temperatures between 31 and 45 °C than wild-type mice, the thermal preference behavior of wild-type and TRPM3 knockout mice was very similar on a surface temperature gradient of 5 to 60 °C [124]. Regarding the other two warmth-sensitive TRP channels, TRPM4 and TRPM5, no studies investigating the functions of the two channels in thermosensation have been reported.
In contrast to the lack of a clear role for any individual TRP channel in the sensation of warmth or heat, the TRPM8 ion channel, which is activated by cool temperatures [70, 85], plays a clear role in determining the thermal preference of mice over the temperature range of non-noxious coolness between 15 and 30 °C [2, 17, 54, 55]. Thus, the only thermally activated TRP ion channel, out of those discussed above, that unambiguously confers thermal sensitivity upon the behavior of an intact animal appears to be the “cool” receptor, TRPM8.
Molecular basis of warmth sensation
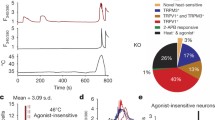
The molecular mechanisms underlying non-noxious warmth sensation remained elusive until we recently demonstrated an essential role for TRPM2 [109]. As noted above, mouse models in which TRPV1, TRPV2, TRPV3, TRPV4, and TRPM3 had been genetically deleted showed no clear deficits in thermal sensation in the range of innocuous warmth between 30 and 40 °C. In addition, the fact that nearly 50% of dorsal root ganglion (DRG) neurons from TRPM3 KO mice still responded to heat, even when TRPV1 is blocked by a potent and selective TRPV1 antagonist, suggests the presence in sensory neurons of an additional thermal sensor or sensors other than TRPM3 or TRPV1 [124]. We set out to discover novel heat-sensitive mechanisms using calcium imaging. Our strategy was first to identify somatosensory neurons expressing TRPV1, TRPV2, TRPV3, TRPV4, or TRPM3, using agonists of these known thermo-TRP channels, and then to focus on the properties of the novel thermally activated neurons that did not express any of these known thermo-TRPs. We found that around 10% of DRG neurons were heat-activated but did not express any known thermo-TRP, and therefore express the proposed novel heat-sensitive mechanism. This finding does not necessarily indicate that the expression of the novel heat-sensitive mechanism is limited to the 10% of DRG neurons, as it is possible that the novel heat-sensitive mechanism could also be co-expressed with other thermo-TRP channels. We therefore tested the responses of DRG neurons to heat stimuli after blocking TRPV1 with AMG9810 and TRPM3 with naringenin, and we found that 46% of DRG neurons responded to heat, suggesting significant co-expression of the novel heat-sensitive mechanism with TRPV1 and TRPM3. Around half of DRG neurons therefore express the novel heat-sensitive mechanism, with around 10% expressing it in isolation, and a further ~ 40% in combination with TRPV1 and/or TRPM3.
In order to better characterize the novel heat-sensitive mechanism, we sought to identify a source of neurons expressing a less complex set of heat-sensitive ion channels than is present in DRG neurons. In autonomic ganglion neurons, both sympathetic and parasympathetic, we found that around half of isolated neurons showed a significant calcium increase in response to heat, but that very few neurons showed any response to agonists of known thermo-TRP channels. Autonomic neurons therefore express the novel heat-sensitive mechanism in isolation, which offers an advantageous preparation for characterizing its properties. Heat-evoked increases of the intracellular calcium concentration were completely abolished by removal of extracellular calcium, and so were caused by an influx of calcium from the extracellular solution. In addition, the calcium influx was reduced but not abolished in the absence of extracellular sodium or in the presence of L-type calcium channel blockers, and was unaffected by the voltage-dependent sodium channel blocker tetrodotoxin or by the TRPV channel blocker ruthenium red. These results indicated that the heat-evoked calcium entry occurred through a mixed sodium and calcium-permeable channel, and that in the presence of sodium, the main current carrier, depolarization of neurons to the threshold for generation of action potentials by voltage-gated calcium channels caused an additional calcium influx. By evaluating the current-voltage relations of autonomic neurons voltage-clamped in the whole-cell configuration at 36 and 47 °C, with all conventional voltage-dependent ion channels blocked, the current-voltage relation of the heat-activated ion channel was shown to be approximately linear, with a reversal potential close to 0 mV. The properties of mixed sodium and calcium permeability and a linear current-voltage relation with a reversal potential close to 0 mV suggested the channel to be a TRP ion channel, or possibly a member of the cyclic-nucleotide-gated (CNG) ion channel family.
To determine the molecular identity of the novel heat-sensitive ion channel, we carried out an analysis of RNA sequencing on a sympathetically derived cell line, MAH cells, which are similar to sympathetic neurons in their thermal responses, in that there are no responses to agonists of known thermo-TRP channels, but the novel heat-sensitive mechanism is present in a significant proportion of cells. When differentiated by a cocktail of growth factors, the heat sensitivity of the MAH cells was found to be reduced. RNA sequencing showed that the only channels expressed in MAH cells, out of all TRP and CNG channels, were TRPC1, C2, C3, V2, M2, M4, and M7. Among these, only TRPM2 has a linear current-voltage relation, a mixed sodium and calcium permeability, and was not upregulated by differentiation. The molecular identity of the novel heat-sensitive ion channel was confirmed in sensory and sympathetic neurons from TRPM2−/− mice, in which the percentage of novel heat-sensitive neurons, and the amplitudes of the residual heat responses in the few remaining heat-responsive neurons, were both greatly reduced [109].
Properties of TRPM2
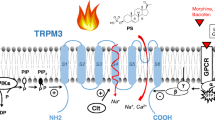
TRPM2 is a fascinating example of a chanzyme, a membrane protein containing both a functional ion channel and an enzymatic domain. The intracellular C terminus contains a nudix hydrolase (NUDT9) homology region, which was named after the mitochondrial ADP-ribose (ADPR) pyrophosphatase NUDT9 and gives TRPM2 the chanzyme designation, although the NUDT9 domain in TRPM2 does not appear to be functional in the enzymatic sense and the channel is activated simply by binding ADPR [45, 72, 74, 87, 98, 115]. The full-length long form TRPM2 protein in humans consists of 1503 amino acid residues (1506 in mouse and 1507 in rat) and is encoded by 32 exons [78, 104]. In addition to the nudix hydrolase domain, the C-terminal contains a coiled-coil region governing channel assembly [72]. The N terminus of the full-length TRPM2 contains four domains of the TRPM homology region, including a calmodulin-binding IQ-like motif providing positive feedback for channel activation, followed by six transmembrane domains with a pore-forming loop domain between S5 and S6 [71, 87, 114]. Partial proteolysis of mitochondrial NUDT9 suggests that NUDT9 enzymes consist of two domains, a C-terminal CORE domain containing segments with ADPR pyrophosphatase activity and an N-terminal CAP domain with the function of enhancing the affinity of the C-terminal CORE domain for ADPR [89]. The nudix hydrolase homology domain (NUDT9-H) was shown to be of importance for normal channel assembly and surface trafficking, because deletion of the domain significantly decreases the membrane expression of TRPM2 [90].
In addition, a variety of splice variants of TRPM2 have been reported. The first splice variant, cloned from HL-60 cells, is characterized by deletions in the N-terminal region (nucleotides 2056–2115) and in the C-terminal region (nucleotides 4318–4419), and is denoted as TRPM2-ΔNΔC. TRPM2-ΔN was shown to be a dysfunctional splice variant of TRPM2, as evidenced by the finding that the sensitivity to H2O2 and ADPR was abolished in HEK293 cells transfected with cDNA of the splice variant [126]. Although the deleted segment overlaps with two IQ-like calmodulin-binding domains and contains two PxxP motifs implicated in protein–protein interactions, the dysfunction was also shown to be unrelated to any of these motifs as removal of each of the two IQ-motifs or deletion of either one or both PxxP motifs caused no functional loss. The findings suggest that the ΔN-stretch may be a spacer segment for other functional sites [60]. TRPM2-ΔC was shown to be unresponsive to ADP-ribose [126]. However, the responses of the splice variant to H2O2 varies among different studies, with some studies showing that TRPM2-ΔC could be activated by H2O2 as efficiently as the wild type [81, 126], while another study shows the ΔC splice variant to be unresponsive to H2O2 [90]. Whether H2O2 directly activates TRPM2, or whether its effect occurs indirectly via a change in ADPR, is therefore uncertain. The studies showing activation of TRPM2-ΔC by H2O2 suggest that the deletion mutant is expressed on the surface membrane, and moreover that the action of H2O2 does not depend on ADPR as mediator [81, 126]. Results showing that deletion of the NUDT9-H domain significantly decreases membrane expression [90] while deletion of the amino acids in the NUDT9-H CAP region of the TRPM2-ΔC channels preserves the membrane expression of TRPM2 [88], suggest that the NUDT9-H CORE domain is involved in the functional expression of TRPM2 on the membrane. The striatal short form (TRPM2-SSF) lacks 214 N-terminal amino acid residues but has intact H2O2-induced Ca2+ influx activity [119]. TRPM2-AS is a short form of TRPM2 consisting of the TRPM2 N terminus and the first two predicted transmembrane domains, resulting from alternative splicing which locates a stop codon (TAG) at the splice junction between exons 16 and 17 [133]. Owing to the lack of the pore region and the C terminus, the splice variant exerts its function in a dominant negative form and has been reported to inhibit TRPM2-mediated calcium influx and thus to reduce susceptibility to cell death caused by exposure to H2O2 [133, 134]. Consistent with this, the short form splice variant was also shown by co-immunoprecipitation to interact with the long form TRPM2 and can exert inhibition of the long form TRPM2 [133]. Although heteromers composed of various combinations of TRP channels have been reported [21, 57], whether TRPM2 interacts with other TRP channels through heteromerization remains unknown.
The activation of TRPM2 by warmth was first shown in HEK293T cells heterologously expressing TRPM2, and the amplitude of the TRPM2 current evoked by ADPR was also shown to be enhanced at elevated temperatures [112]. Beta-NAD+- and cyclic-ADP-ribose evoke little TRPM2 current at 25 °C, but a temperature of 40 °C greatly enhances the currents evoked by beta-NAD+- and cyclic-ADP-ribose [112]. Although the amplitude of TRPM2 current can be enhanced in the presence of beta-NAD+- and cyclic-ADP-ribose, the temperature threshold of TRPM2 was reported to be unaffected by the presence of beta-NAD+- or cyclic-ADP-ribose [112]. In contrast, H2O2 appears to sensitize the heat responses mediated by TRPM2 by lowering the temperature threshold of activation to the range around body temperature both in a transient expression system and in macrophages [49]. The thermosensitivity of TRPM2 and the effect of H2O2 in sensitizing the thermal responses of TRPM2 were also demonstrated in sensory and autonomic neurons [109].
TRPM2 is widely expressed in the central and peripheral nervous system [23], both in nonneuronal microglial cells and in neurons [12, 22]. In addition, a relatively high level of TRPM2 expression was shown in a variety of immune cells [23], including neutrophils [32,33,34, 38, 61, 84], monocytes [87, 127, 131], macrophages [49, 137], dendritic cells [84, 106], mast cell [82], and lymphocytes [4, 6, 94]. The expression pattern of TRPM2 and its sensitivity to temperatures in the range of mammalian body temperature suggest a potential role in thermoregulation, in the immune system, and in inflammatory responses.
TRPM2 is responsible for the detection of non-noxious warmth
In view of the expression of TRPM2 in novel heat-sensitive neurons, we investigated whether TRPM2 might be responsible for non-noxious warmth preference [109]. The thermal preference of wild-type adult male mice for a temperature around 33 °C has been well documented with the two-plate thermal preference test in a number of studies [43, 73, 91]. We used this system with one control plate at 33 °C, the preferred temperature, while the temperature of the test plate was adjusted to cooler or warmer temperatures. The test and control plates were reversed after 30 min to control for any influence of environmental cues. The results showed that adult male wild-type mice avoided the non-noxious warm temperature of 38 °C, in agreement with the results previously published [43, 73, 91], while TRPM2 knockout mice showed no avoidance of 38 °C [109]. Overall, deletion of TRPM2 caused mice to prefer warmer temperatures over a wide thermal range from 23 to above 38 °C, suggesting that TRPM2 is activated by warmth and creates an aversive signal that drives male mice towards cooler temperatures [109]. Thermal behavior may therefore be thought of as a balance between an aversive “warm” signal, created by TRPM2, and an aversive “cool” signal, created by TRPM8 as discussed above [2, 17, 54, 55], with the optimal environmental temperature achieved when the two signals are in balance.
Sex differences in thermal preference
In adult wild-type male mice, a preference for 33 °C over cooler or warmer floor temperatures has been well documented [43, 73, 91], but female mice have a distinct preference for warmer temperatures [47, 73]. Altered female behaviors in response to different ambient temperatures, usually a slightly warmer and less prominent thermal preference than male mice, were also observed [24, 25]. In humans, women prefer a warmer ambient temperature than men [5, 48] and also on average have a cooler peripheral skin temperature than men, along with a warmer core temperature [52]. These differences are therefore consistent across labs, paradigms, and species, but the reasons for the influence of gender on thermal behavior remains both interesting and unknown. Is the difference driven by sex hormones or by other genetic factors? Is it due to a difference in peripheral thermal perception, perhaps driven by sex differences in TRPM2 and/or TRPM8, or is it attributable to other factors?
Participation of TRPM2 in central thermal sensation
In view of the role of TRPM2 in sensing ambient warm temperatures, a possible role for TRPM2 is attractive in the sensation of, and perhaps also in the control of, normal body warmth. TRPM2 was found to be expressed in a subpopulation of neurons in the preoptic area of the hypothalamus, which is known to play an important role in the sensation of central temperatures and in whole-body thermoregulation [103]. In calcium imaging experiments, these neurons were found not to be active at the normal body temperature of 37 °C, in both cell cultures and in brain slices, but to be activated by small temperature elevations, to 38°C or above, in brain slices. Consistent with this, normal body temperature was unaffected by genetic deletion of TRPM2, and mild fever induced by injection of a small dose of PGE2 into the hypothalamus was also unaffected. However, a larger increase in body temperature was noted in TRPM2 knockout mice after injecting a higher dose of PGE2 or other pyrogenic stimuli into the hypothalamus, suggesting that TRPM2 plays a role as a “brake” which prevents the development of an excessive fever response. Chemogenetic activation and inhibition of hypothalamic TRPM2-expressing neurons in vivo caused a decrease and increase of body temperature, respectively, showing that these neurons are potently connected to neural circuits involved in the control of body temperature [103]. Overall, this fascinating work suggests that TRPM2 does not play a central role in the maintenance of normal body temperature nor in mild fever but appears instead to act as an “emergency response system” which is able to bring into play potent thermoregulatory responses in order to reduce body temperature when it rises to dangerous levels.
Role of TRPM2 in the autonomic nervous system
Neurons of the autonomic nervous system, both sympathetic and parasympathetic, express a warm-sensory function mediated by TRPM2, as described above [109]. This finding is surprising because the autonomic nervous system has been traditionally viewed as carrying out a purely motor function, and the idea that it may have an autonomous sensory function has not, to the best of our knowledge, been considered before.
The prevailing concept of thermoregulation holds that the peripheral somatosensory system detects the external environment temperature and sends information to the hypothalamus, where it is integrated with information on core body temperature detected in the preoptic area. The sympathetic nervous system then serves as the output pathway that transmits the integrated thermal command to control the vasomotor tone of surface blood vessels, the activity of brown adipose tissue, and sweating [76]. However, the model ignores a possible effect of temperature acting directly and locally on tissues. For example, local warming has long been known to cause local vasodilation [110], an effect that has been attributed to a reflex release of vasodilator peptides such as CGRP from activated sensory nerve endings. However, one study showed that when conduction of action potentials in sensory nerves was blocked with anesthetic cream, the steady-state vasodilation by local warming up to 42 °C was not different from control, but that the vasodilation was significantly reduced following inhibition of exocytosis from postganglionic sympathetic neurons with bretylium [7]. This result suggests that the peripheral sympathetic nervous system is more important than the somatosensory nervous system for the steady-state vasodilation evoked by local warming. It may be counter-intuitive to believe that postganglionic sympathetic neurons have an important thermosensory role in the vasodilation evoked by local warming, but evidence for this view is growing because the inhibition of local warming-evoked vasodilation by bretylium has now been shown in many studies [39, 40, 42, 111]. It would be of great interest to investigate a potential physiological role for TRPM2 as a thermosensor in the autonomic nervous system.
Role of TRPM2 in the immune system
TRPM2 is widely expressed in the immune system [96], and the sensitivity of TRPM2 to oxidative stress suggests a potential involvement of TRPM2 in inflammatory responses [30, 126]. The oxidative stress-induced Ca2+ influx through TRPM2 initiates downstream reactions essential for chemokine production and the resultant inflammatory responses. The crucial role of TRPM2 in inflammatory responses is supported by the impairment of H2O2-induced production of macrophage inflammatory protein-2 (MIP2, also known as CXCL2) in monocytes from TRPM2 knockout mice [131]. TRPM2 was shown to participate in the enhancement of bactericidal activity by lysophosphatidylcholine (LPC) via a signaling pathway involving glycine receptor α2, TRPM2, and p38 mitogen-activated protein kinases [41]. Furthermore, TRPM2 knockout mice were shown to be highly susceptible to infection with Listeria monocytogenes (Lm) and exhibited impaired innate immunity with reduced level of cytokines IL-12 and IFNγ after Lm infection [53]. These studies suggest that TRPM2 plays an enhancing role in inflammatory responses.
On the other hand, one study evaluated the function of TRPM2 in a model of endotoxin-induced lung inflammation and found increased release of chemokines and proinflammatory cytokines, including tumor necrosis factor, the chemokine CXCL2 and interleukin 6, in the lungs of TRPM2 knockout mice. These results suggest that TRPM2 plays a negative feedback role in dampening the inflammatory responses induced by oxidative stress [18]. Another study showed that TRPM2 knockout mice chronically infected with Helicobacter pylori exhibited increased gastric inflammation and greater macrophage production of inflammatory mediators and macrophage M1 polarization. These results, in contrast, are in favor of an inhibitory role of TRPM2 in inflammatory responses [3]. The exact role of TRPM2 in inflammatory responses warrants further clarification.
Role of TRPM2 in pain
A role for TRPM2 in the pathogenesis of inflammatory and neuropathic pain is suggested from the expression of TRPM2 both in the peripheral nervous system and in the immune system, together with the fact that TRPM2 is activated by oxidative stress. One study showed that in carrageenan-induced inflammatory pain and sciatic nerve injury-induced neuropathic pain models, mechanical allodynia and thermal hyperalgesia were attenuated in TRPM2 knockout mice [31]. Another study showed that the severity of pathological pain conditions are significantly reduced in TRPM2 knockout mice; the models investigated include acetic acid-induced writhing behavior, mechanical allodynia in the monosodium iodoacetate-induced osteoarthritis pain model, the experimental autoimmune encephalomyelitis model, the paclitaxel-induced peripheral neuropathy model, and the streptozotocin-induced painful diabetic neuropathy model [102]. Furthermore, the trinitrobenzene sulfonic acid-induced visceral hypersensitivity was significantly reduced in TRPM2 knockout mice, while the enhanced visceromotor response to noxious colorectal distention induced in colitis model was restored to the control level after treatment with econazole, a TRPM2 inhibitor [69]. These results show that TRPM2 promotes the development of pathological pain states. Selective blockers of TRPM2 may find a future use as analgesics.
Role of TRPM2 in insulin secretion
TRPM2 is expressed in pancreatic beta cells, which under normal circumstances are not exposed to significant changes in temperature. Reactive oxygen species such as H2O2 could, however, modulate TRPM2 and therefore influence the secretion of insulin. In isolated pancreatic islet cells, H2O2 induces both a greater calcium increase and enhanced insulin secretion when compared to its effect in cells from TRPM2 knockout mice [50, 117]. A physiological role for TRPM2 is suggested from the observations that TRPM2 knockout mice show attenuated insulin secretion and higher blood glucose levels in glucose tolerance tests [51, 118]. TRPM2 may therefore be an important participant in the physiology of glucose homeostasis, though the details of the mechanism are yet to be fully elucidated.
Conclusion
Over the last decade, we have gained a much deeper understanding of the role of TRPM2 in thermosensation and thermoregulation, in the immune system and in pain, but there are still many unresolved questions. Why is thermosensation sex-dependent, and is the difference driven by TRPM2? Does TRPM2 contribute to the crucial mechanism that maintains basal core body temperature at around 37 °C? Present evidence suggests that it does not—but is TRPM2 instead involved in initiating or regulating fever? TRPM2 appears to be implicated in the responses of the immune system, which are known to be strongly temperature-dependent, but the mechanistic details are unclear. There are conflicting results regarding the role of TRPM2 in inflammatory responses. All these questions are of crucial importance and need further investigation.
References
Abriel H, Syam N, Sottas V, Amarouch MY, Rougier JS (2012) TRPM4 channels in the cardiovascular system: physiology, pathophysiology, and pharmacology. Biochem Pharmacol 84:873–881
Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–208
Beceiro S, Radin JN, Chatuvedi R, Piazuelo MB, Horvarth DJ, Cortado H, Gu Y, Dixon B, Gu C, Lange I, Koomoa DL, Wilson KT, Algood HM, Partida-Sanchez S (2017) TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol 10:493–507
Beck A, Kolisek M, Bagley LA, Fleig A, Penner R (2006) Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J : Off Publ Fed Am Soc Exp Biol 20:962–964
Beshir MY, Ramsey JD (1981) Comparison between male and female subjective estimates of thermal effects and sensations. Appl Ergon 12:29–33
Buelow B, Song Y, Scharenberg AM (2008) The poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem 283:24571–24583
Carter SJ, Hodges GJ (2011) Sensory and sympathetic nerve contributions to the cutaneous vasodilator response from a noxious heat stimulus. Exp Physiol 96:1208–1217
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Cesare P, McNaughton P (1996) A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A 93:15435–15439
Chung KK, Freestone PS, Lipski J (2011) Expression and functional properties of TRPM2 channels in dopaminergic neurons of the substantia nigra of the rat. J Neurophysiol 106:2865–2875
Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ (2004) TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem 279:21569–21575
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524
Clapham DE, Julius D, Montell C, Schultz G (2005) International union of pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57:427–450
Croze S, Duclaux R, Kenshalo DR (1976) The thermal sensitivity of the polymodal nociceptors in the monkey. J Physiol 263:539–562
Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A (2007) TRPM8 is required for cold sensation in mice. Neuron 54:371–378
Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM, Malik AB (2012) The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 13:29–34
Dodt E, Zotterman Y (1952) Mode of action of warm receptors. Acta Physiol Scand 26:345–357
Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O'Brien PC (1993) Cool, warm, and heat-pain detection thresholds: testing methods and inferences about anatomic distribution of receptors. Neurology 43:1500–1508
Fischer MJ, Balasuriya D, Jeggle P, Goetze TA, McNaughton PA, Reeh PW, Edwardson JM (2014) Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Archiv : Eur J Physiol 466:2229–2241
Fonfria E, Mattei C, Hill K, Brown JT, Randall A, Benham CD, Skaper SD, Campbell CA, Crook B, Murdock PR, Wilson JM, Maurio FP, Owen DE, Tilling PL, and McNulty S. TRPM2 is elevated in the tMCAO stroke model, transcriptionally regulated, and functionally expressed in C13 microglia. J Recept Signal Transduct Res 26: 179–198, (2006), TRPM2 Is Elevated in the tMCAO Stroke Model, Transcriptionally Regulated, and Functionally Expressed in C13 Microglia
Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26:159–178
Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP (2012) Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One 7:e32799
Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP (2009) Some like it hot: mouse temperature preferences in laboratory housing. Appl Anim Behav Sci 116:279–285
González-Duarte A, Lem-Carrillo M, and Guerrero-Torres L. (2016) Normative values of quantitative sensory testing in Hispanic Latino population. Brain and Behavior 6: n/a-n/a, 6, e00466
Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278:21493–21501
Guinamard R, Demion M, and Launay P (2010) Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda, MD 25: 155–164
Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci : Off J Soc Neurosci 22:6408–6414
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173
Haraguchi K, Kawamoto A, Isami K, Maeda S, Kusano A, Asakura K, Shirakawa H, Mori Y, Nakagawa T, Kaneko S (2012) TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci : Off J Soc Neurosci 32:3931–3941
Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jungling E, Zitt C, Luckhoff A (2003) Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J 371:1045–1053
Heiner I, Eisfeld J, Luckhoff A (2003) Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 33:533–540
Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jungling E, Luckhoff A (2006) Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J 398:225–232
Hensel H, Iggo A (1971) Analysis of cutaneous warm and cold fibres in primates. Pflugers Arch 329:1–8
Hensel H, Iggo A, Witt I (1960) A quantitative study of sensitive cutaneous thermoreceptors with C afferent fibres. J Physiol 153:113–126
Hensel H, Zotterman Y (1951) Quantitative Beziehungen zwischen der Entladung einzelner Kältefasern und der Temperatur. Acta Physiol Scand 23:291–319
Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y, Mori Y, Shimizu S (2013) Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc Res 97:271–281
Hodges GJ, Kosiba WA, Zhao K, Johnson JM (2009) The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Phys Heart Circ Phys 296:H51–H56
Hodges GJ, Kosiba WA, Zhao K, and Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol (Bethesda, MD : 1985) 105: 233–240, 2008
Hong CW, Kim TK, Ham HY, Nam JS, Kim YH, Zheng H, Pang B, Min TK, Jung JS, Lee SN, Cho HJ, Kim EJ, Hong IH, Kang TC, Lee J, Oh SB, Jung SJ, Kim SJ, and Song DK. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol (Baltimore, MD : 1950) 184: 4401–4413, 2010
Houghton BL, Meendering JR, Wong BJ, Minson CT (2006) Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572:811–820
Huang SM, Li X, Yu Y, Wang J, Caterina MJ (2011) TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 7(37):1744-8069-7-37
Iggo A (1969) Cutaneous thermoreceptors in primates and sub-primates. J Physiol 200:403–430
Iordanov I, Mihalyi C, Toth B, Csanady L (2016) The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity. elife 5
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384
Kaikaew K, Steenbergen J, Themmen APN, Visser JA, Grefhorst A (2017) Sex difference in thermal preference of adult mice does not depend on presence of the gonads. Biol Sex Differ 8(24):24
Karjalainen S (2007) Gender differences in thermal comfort and use of thermostats in everyday thermal environments. Build Environ 42:1594–1603
Kashio M, Sokabe T, Shintaku K, Uematsu T, Fukuta N, Kobayashi N, Mori Y, Tominaga M (2012) Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc Natl Acad Sci U S A 109:6745–6750
Kashio M, Tominaga M (2015) Redox signal-mediated enhancement of the temperature sensitivity of transient receptor potential melastatin 2 (TRPM2) elevates glucose-induced insulin secretion from pancreatic islets. J Biol Chem 290:12435–12442
Kashio M, Tominaga M (2017) The TRPM2 channel: a thermo-sensitive metabolic sensor. Channels (Austin) 11:426–433
Kim H, Richardson C, Roberts J, Gren L, Lyon JL (1998) Cold hands, warm heart. Lancet 351:1492
Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, Shapland E, Kucera G, Mogan J, Humann J, Lenz LL, Morrison AD, Perraud AL (2011) Transient receptor potential melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci U S A 108:11578–11583
Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD (2010) TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150:340–350
Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD (2013) A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. JNeurosci 33:2837–2848
Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493:596–606
Kobori T, Smith GD, Sandford R, Edwardson JM (2009) The transient receptor potential channels TRPP2 and TRPC1 form a heterotetramer with a 2:2 stoichiometry and an alternating subunit arrangement. J Biol Chem 284:35507–35513
Kolisek M, Beck A, Fleig A, Penner R (2005) Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 18:61–69
Kowase T, Nakazato Y, Yoko OH, Morikawa A, Kojima I (2002) Immunohistochemical localization of growth factor-regulated channel (GRC) in human tissues. Endocr J 49:349–355
Kuhn FJ, Kuhn C, Naziroglu M, Luckhoff A (2009) Role of an N-terminal splice segment in the activation of the cation channel TRPM2 by ADP-ribose and hydrogen peroxide. Neurochem Res 34:227–233
Lange I, Penner R, Fleig A, Beck A (2008) Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium 44:604–615
Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, Huang M, Lin JH, Feder JN, Janovitz EB, Levesque PC, Blanar MA (2003) Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 278:20890–20897
Leffler A, Linte RM, Nau C, Reeh P, Babes A (2007) A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur J Neurosci 26:12–22
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ (2010) TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol 11:232–239
Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C (2010) Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 151:598–605
Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD (2010) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150:439–450
Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M (2009) TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch 458:1093–1102
Matsumoto K, Takagi K, Kato A, Ishibashi T, Mori Y, Tashima K, Mitsumoto A, Kato S, Horie S (2016) Role of transient receptor potential melastatin 2 (TRPM2) channels in visceral nociception and hypersensitivity. Exp Neurol 285:41–50
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58
Mei ZZ, Mao HJ, Jiang LH (2006) Conserved cysteine residues in the pore region are obligatory for human TRPM2 channel function. Am J Physiol Cell Physiol 291:C1022–C1028
Mei ZZ, Xia R, Beech DJ, Jiang LH (2006) Intracellular coiled-coil domain engaged in subunit interaction and assembly of melastatin-related transient receptor potential channel 2. J Biol Chem 281:38748–38756
Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A (2011) TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat Commun 2:369
Montell C, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108:595–598
Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307:1468–1472
Morrison SF (2016) Central control of body temperature. F1000Research 5
Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829–838
Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N (1998) Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 54:124–131
Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, Jiang S, Seymour VA, McKeown L, Kumar B, Harteneck C, O'Regan D, Wheatcroft SB, Kearney MT, Jones C, Porter KE, Beech DJ (2010) Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res 106:1507–1515
Nilius B, Prenen J, Janssens A, Voets T, Droogmans G (2004) Decavanadate modulates gating of TRPM4 cation channels. J Physiol 560:753–765
Numata T, Sato K, Christmann J, Marx R, Mori Y, Okada Y, Wehner F (2012) The DeltaC splice-variant of TRPM2 is the hypertonicity-induced cation channel in HeLa cells, and the ecto-enzyme CD38 mediates its activation. J Physiol 590:1121–1138
Oda S, Uchida K, Wang X, Lee J, Shimada Y, Tominaga M, Kadowaki M (2013) TRPM2 contributes to antigen-stimulated Ca(2)(+) influx in mucosal mast cells. Pflugers Archiv : Eur J Physiol 465:1023–1030
Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ (2011) TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci : Off J Soc Neurosci 31:11425–11436
Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, Mousseau BJ, Sumoza-Toledo A, Bhagat H, Walseth TF, Guse AH, and Lund FE (2007) Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol (Baltimore, MD : 1950) 179: 7827–7839
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411:595–599
Perraud AL, Schmitz C, Scharenberg AM (2003) TRPM2 Ca2+ permeable cation channels: from gene to biological function. Cell Calcium 33:519–531
Perraud AL, Shen B, Dunn CA, Rippe K, Smith MK, Bessman MJ, Stoddard BL, Scharenberg AM (2003) NUDT9, a member of the Nudix hydrolase family, is an evolutionarily conserved mitochondrial ADP-ribose pyrophosphatase. J Biol Chem 278:1794–1801
Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM (2005) Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 280:6138–6148
Pogorzala LA, Mishra SK, Hoon MA (2013) The cellular code for mammalian thermosensation. J Neurosci : Off J Soc Neurosci 33:5533–5541
Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A 100:15166–15171
Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM (2008) TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci : Off J Soc Neurosci 28:6231–6238
Roedding AS, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ (2012) Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord 14:151–161
Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B (2006) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 123:231–243
Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293:1327–1330
Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, Chou M, Middleton R, Kaczorowski G, Jones AB (2002) Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J PharmacolExpTher 303:1052–1060
Shen BW, Perraud AL, Scharenberg A, Stoddard BL (2003) The crystal structure and mutational analysis of human NUDT9. J Mol Biol 332:385–398
Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB (2000) The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 129:227–230
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418:186–190
Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE (2006) Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem 281:29897–29904
So K, Haraguchi K, Asakura K, Isami K, Sakimoto S, Shirakawa H, Mori Y, Nakagawa T, Kaneko S (2015) Involvement of TRPM2 in a wide range of inflammatory and neuropathic pain mouse models. J Pharmacol Sci 127:237–243
Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J (2016) The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353:1393–1398
Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA (2002) Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A 99:16899–16903
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702
Sumoza-Toledo A, Lange I, Cortado H, Bhagat H, Mori Y, Fleig A, Penner R, Partida-Sanchez S (2011) Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J : Off Publ Fed Am Soc Exp Biol 25:3529–3542
Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, Kinet JP, Fleig A, Yamada T, Penner R (2006) A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol 69:1413–1420
Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438:1022–1025
Tan CH, McNaughton PA (2016) The TRPM2 ion channel is required for sensitivity to warmth. Nature 536:460–463
Taylor WF, Johnson JM, O'Leary D, Park MK (1984) Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol Respir Environ Exerc Physiol 57:191–196
Tew GA, Saxton JM, Klonizakis M, Moss J, Ruddock AD, and Hodges GJ (2011) Aging and aerobic fitness affect the contribution of noradrenergic sympathetic nerves to the rapid cutaneous vasodilator response to local heating. J Appl Physiol (Bethesda, MD : 1985) 110: 1264–1270
Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25:1804–1815
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543
Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, Miller BA (2006) Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem 281:9076–9085
Toth B, Iordanov I, Csanady L (2014) Putative chanzyme activity of TRPM2 cation channel is unrelated to pore gating. Proc Natl Acad Sci U S A 111:16949–16954
Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P (2002) Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. NatNeurosci 5:546–551
Uchida K, Tominaga M (2014) The role of TRPM2 in pancreatic beta-cells and the development of diabetes. Cell Calcium 56:332–339
Uchida K, Tominaga M (2011) TRPM2 modulates insulin secretion in pancreatic beta-cells. Islets 3:209–211
Uemura T, Kudoh J, Noda S, Kanba S, Shimizu N (2005) Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun 328:1232–1243
Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B (2005) Comparison of functional properties of the Ca2+−activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37:267–278
van den Bosch GE, van Dijk M, Tibboel D, Valkenburg AJ (2017) Thermal quantitative sensory testing in healthy Dutch children and adolescents standardized test paradigm and Dutch reference values. BMC Pediatr 17(77)
Vlachova V, Teisinger J, Susankova K, Lyfenko A, Ettrich R, Vyklicky L (2003) Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci : Off J Soc Neurosci 23:1340–1350
Voets T (2012) Quantifying and modeling the temperature-dependent gating of TRP channels. Rev Physiol Biochem Pharmacol 162:91–119
Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T (2011) TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70:482–494
Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 10:1421–1430
Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277:23150–23156
Wehrhahn J, Kraft R, Harteneck C, and Hauschildt S (2010) Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol (Baltimore, MD : 1950) 184: 2386–2393
Xiong J, Lian Z, Zhou X, You J, Lin Y (2015) Investigation of gender difference in human response to temperature step changes. Physiol Behav 151:426–440
Xu H, Delling M, Jun JC, Clapham DE (2006) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9:628–635
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–186
Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y (2008) TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14:738–747
Yang G, Luo Y, Baad-Hansen L, Wang K, Arendt-Nielsen L, Xie QF, Svensson P (2013) Ethnic differences in oro-facial somatosensory profiles-quantitative sensory testing in Chinese and Danes. J Oral Rehabil 40:844–853
Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA (2003) A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 278:16222–16229
Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun SC, Penn L, Barber DL, Stahl R, Carey DJ, Cheung JY, Miller BA (2006) TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol 290:C1146–C1159
Zotterman Y (1935) Action potentials in the glossopharyngeal nerve and in the chorda tympani. Skandinavisches Archiv Für Physiologie 72:73–77
Zotterman Y (1936) Specific action potentials in the lingual nerve of cat. Skandinavisches Archiv Für Physiologie 75:105–119
Zou J, Ainscough JF, Yang W, Sedo A, Yu SP, Mei ZZ, Sivaprasadarao A, Beech DJ, and Jiang LH (2013) A differential role of macrophage TRPM2 channels in Ca(2)(+) signaling and cell death in early responses to H(2)O(2). Am J Physiol Cell Physiol 305: C61–69
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue on Thermal biology in Pflügers Archiv – European Journal of Physiology
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tan, CH., McNaughton, P.A. TRPM2 and warmth sensation. Pflugers Arch - Eur J Physiol 470, 787–798 (2018). https://doi.org/10.1007/s00424-018-2139-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2139-7