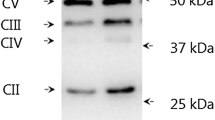
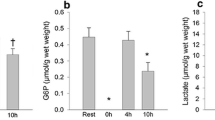
Abstract
Pyruvate dehydrogenase (PDH) is the gateway enzyme for carbohydrate-derived pyruvate feeding into the TCA cycle. PDH may play a central role in regulating substrate shifts during exercise, but the influence of training state on PDH regulation during exercise is not fully elucidated. The purpose of this study was to investigate the impact of training state on post-translational regulation of PDHa activity during submaximal and exhaustive exercise. Eight untrained and nine endurance exercise-trained healthy male subjects performed incremental exercise on a cycle ergometer: 40 min at 50% incremental peak power output (IPPO), 10 min at 65% (IPPO), followed by 80% (IPPO) until exhaustion. Trained subjects had higher (P < 0.05) PDH-E1α, PDK1, PDK2, PDK4, and PDP1 protein content as well as PDH phosphorylation and PDH acetylation. Exercising at the same relative intensity led to similar muscle PDH activation in untrained and trained subjects, whereas PDHa activity at exhaustion was higher (P < 0.05) in trained than untrained. Furthermore, exercise induced similar PDH dephosphorylation in untrained and trained subjects, while PDH acetylation was increased (P < 0.05) only in trained subjects. In conclusion, PDHa activity and PDH dephosphorylation were well adjusted to the relative exercise intensity during submaximal exercise. In addition, higher PDHa activity in trained than untrained at exhaustion seemed related to differences in glycogen utilization rather than differences in PDH phosphorylation and acetylation state, although site-specific contributions cannot be ruled out.








Similar content being viewed by others
References
Bergmeyer HU, Moellering H (1965) Acylphosphate: D-glucose-6-phosphotransferase. Biochem Z 343:97–102
Bergstrom J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616
Bienso RS, Knudsen JG, Brandt N, Pedersen PA, Pilegaard H (2014) Effects of IL-6 on pyruvate dehydrogenase regulation in mouse skeletal muscle. Pflugers Arch 466:1647–1657
Bienso RS, Olesen J, Gliemann L, Schmidt JF, Matzen MS, Wojtaszewski JF, Hellsten Y, Pilegaard H (2015) Effects of exercise training on regulation of skeletal muscle glucose metabolism in elderly men. J Gerontol A Biol Sci Med Sci 70:866–872
Bienso RS, Olesen J, van Hauen L, Meinertz S, Halling JF, Gliemann L, Plomgaard P, Pilegaard H (2015) Exercise-induced AMPK and pyruvate dehydrogenase regulation is maintained during short-term low-grade inflammation. Pflugers Arch 467:341–350
Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM (1998) Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 329(Pt 1):191–196
Brandauer J, Andersen MA, Kellezi H, Risis S, Frosig C, Vienberg SG, Treebak JT (2015) AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD. Front Physiol 6:85
Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, Gibala MJ (2008) Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586:151–160
Cederblad G, Carlin JI, Constantin-Teodosiu D, Harper P, Hultman E (1990) Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Anal Biochem 185:274–278
Christensen EH, Hansen O (1939) Arbeitsfähigkeit und Ernährung. Skandinavisches Arch Physiol 81:160–171. doi:10.1111/j.1748-1716.1939.tb01320.x
Consitt LA, Saxena G, Saneda A, Houmard JA (2016) Age-related impairments in skeletal muscle PDH phosphorylation and plasma lactate are indicative of metabolic inflexibility and the effects of exercise training. Am J Physiol Endocrinol Metab 311:E145–E156
Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E (1991) Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiol Scand 143:367–372
Constantin-Teodosiu D, Cederblad G, Hultman E (1991) A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Anal Biochem 198:347–351
Evans WJ, Phinney SD, Young VR (1982) Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14:101–102
Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, Gu TL, Aguiar M, Lonning S, Chen H, Mohammadi M, Britton LM, Garcia BA, Aleckovic M, Kang Y, Kaluz S, Devi N, Van Meir EG, Hitosugi T, Seo JH, Lonial S, Gaddh M, Arellano M, Khoury HJ, Khuri FR, Boggon TJ, Kang S, Chen J (2014) Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell 53:534–548
Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK (2004) Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab 287:E1189–E1194
Gibala MJ, MacLean DA, Graham TE, Saltin B (1998) Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Phys 275:E235–E242
Graham TE, Saltin B (1989) Estimation of the mitochondrial redox state in human skeletal muscle during exercise. J Appl Physiol 66:561–566
Green HJ, Jones S, Ball-Burnett ME, Smith D, Livesey J, Farrance BW (1991) Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol 70:2032–2038
Guan KL, Xiong Y (2011) Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci 36:108–116
Gudiksen A, Schwartz CL, Bertholdt L, Joensen E, Knudsen JG, Pilegaard H (2016) Lack of skeletal muscle IL-6 affects pyruvate dehydrogenase activity at rest and during prolonged exercise. PLoS One 11:e0156460
Harris RA, Bowker-Kinley MM, Huang B, Wu P (2002) Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzym Regul 42:249–259
Henriksson J (1977) Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol 270:661–675
Hermansen L, Hultman E, Saltin B (1967) Muscle glycogen during prolonged severe exercise. Acta Physiol Scand 71:129–139
Holloszy JO (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242:2278–2282
Holloszy JO, Booth FW (1976) Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38:273–291
Howlett RA, Parolin ML, Dyck DJ, Hultman E, Jones NL, Heigenhauser GJ, Spriet LL (1998) Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Am J Phys 275:R418–R425
Jansson E, Kaijser L (1987) Substrate utilization and enzymes in skeletal muscle of extremely endurance-trained men. J Appl Physiol 62:999–1005
Jing E, O'Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, Kahn CR (2013) Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62:3404–3417
Kelley DE (2005) Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 115:1699–1702
Kelley DE, Mandarino LJ (2000) Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49:677–683
Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB (2004) AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 320:449–454
Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B (1993) Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol 469:459–478
Kiilerich K, Adser H, Jakobsen AH, Pedersen PA, Hardie DG, Wojtaszewski JF, Pilegaard H (2010) PGC-1alpha increases PDH content but does not change acute PDH regulation in mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol 299:R1350–R1359
Kiilerich K, Birk JB, Damsgaard R, Wojtaszewski JF, Pilegaard H (2008) Regulation of PDH in human arm and leg muscles at rest and during intense exercise. Am J Physiol Endocrinol Metab 294:E36–E42
Kiilerich K, Gudmundsson M, Birk JB, Lundby C, Taudorf S, Plomgaard P, Saltin B, Pedersen PA, Wojtaszewski JF, Pilegaard H (2010) Low muscle glycogen and elevated plasma free fatty acid modify but do not prevent exercise-induced PDH activation in human skeletal muscle. Diabetes 59:26–32
Kiilerich K, Ringholm S, Bienso RS, Fisher JP, Iversen N, van Hall G, Wojtaszewski JFP, Saltin B, Lundby C, Calbet JAL, Pilegaard H (2011) Exercise-induced pyruvate dehydrogenase activation is not affected by 7 days of bed rest. J Appl Physiol 111:751–757
Klein DK, Pilegaard H, Treebak JT, Jensen TE, Viollet B, Schjerling P, Wojtaszewski JF (2007) Lack of AMPKalpha2 enhances pyruvate dehydrogenase activity during exercise. Am J Physiol Endocrinol Metab 293:E1242–E1249
Klein S, Coyle EF, Wolfe RR (1994) Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am J Phys 267:E934–E940
Korotchkina LG, Khailova LS, Severin SE (1995) The effect of phosphorylation on pyruvate dehydrogenase. FEBS Lett 364:185–188
Korotchkina LG, Patel MS (2001) Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem 276:37223–37229
LeBlanc PJ, Howarth KR, Gibala MJ, Heigenhauser GJ (2004) Effects of 7 wk of endurance training on human skeletal muscle metabolism during submaximal exercise. J Appl Physiol 97:2148–2153
LeBlanc PJ, Peters SJ, Tunstall RJ, Cameron-Smith D, Heigenhauser GJ (2004) Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J Physiol 557:559–570
Li Y, Dash RK, Kim J, Saidel GM, Cabrera ME (2009) Role of NADH/NAD+ transport activity and glycogen store on skeletal muscle energy metabolism during exercise: in silico studies. Am J Physiol Cell Physiol 296:C25–C46
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE (2005) Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568:665–676
Mourtzakis M, Saltin B, Graham T, Pilegaard H (2006) Carbohydrate metabolism during prolonged exercise and recovery: interactions between pyruvate dehydrogenase, fatty acids, and amino acids. J Appl Physiol 100:1822–1830
Ozden O, Park SH, Wagner BA, Yong SH, Zhu Y, Vassilopoulos A, Jung B, Buettner GR, Gius D (2014) SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med 76:163–172
Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJ (1999) Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Phys 277:E890–E900
Passonneau JV, Lauderdale VR (1974) A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60:405–412
Peronnet F, Massicotte D (1991) Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16:23–29
Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM (1996) Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Phys 270:E265–E272
Pilegaard H, Birk JB, Sacchetti M, Mourtzakis M, Hardie DG, Stewart G, Neufer PD, Saltin B, van Hall G, Wojtaszewski JF (2006) PDH-E1alpha dephosphorylation and activation in human skeletal muscle during exercise: effect of intralipid infusion. Diabetes 55:3020–3027
Putman CT, Spriet LL, Hultman E, Lindinger MI, Lands LC, McKelvie RS, Cederblad G, Jones NL, Heigenhauser GJ (1993) Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am J Phys 265:E752–E760
Randle PJ (1964) Fuel and power in the control of carbohydrate metabolism in mammalian muscle. Symp Soc Exp Biol 18:129–155
Randle PJ (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14:263–283
Ringholm S, Olesen J, Pedersen JT, Brandt CT, Halling JF, Hellsten Y, Prats C, Pilegaard H (2013) Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1alpha. Exp Gerontol 48:1311–1318
Ronsen O, Holm K, Staff H, Opstad PK, Pedersen BK, Bahr R (2001) No effect of seasonal variation in training load on immuno-endocrine responses to acute exhaustive exercise. Scand J Med Sci Sports 11:141–148
Sahlin K (1985) NADH in human skeletal muscle during short-term intense exercise. Pflugers Arch 403:193–196
Sato S, Shirato K, Tachiyashiki K, Imaizumi K (2011) Muscle plasticity and beta(2)-adrenergic receptors: adaptive responses of beta(2)-adrenergic receptor expression to muscle hypertrophy and atrophy. J Biomed Biotechnol 2011:729598
Schwer B, North BJ, Frye RA, Ott M, Verdin E (2002) The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol 158:647–657
Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D (2004) Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting. J Appl Physiol 96:2082–2087
St Amand TA, Spriet LL, Jones NL, Heigenhauser GJ (2000) Pyruvate overrides inhibition of PDH during exercise after a low-carbohydrate diet. Am J Physiol Endocrinol Metab 279:E275–E283
Sugden MC, Holness MJ (2003) Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 284:E855–E862
Sugden MC, Holness MJ (2006) Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem 112:139–149
Trumbo P, Schlicker S, Yates AA, Poos M (2002) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 102:1621–1630
Vargas-Ortiz K, Perez-Vazquez V, Diaz-Cisneros FJ, Figueroa A, Jimenez-Flores LM, Rodriguez-DelaRosa G, Macias MH (2015) Aerobic training increases expression levels of SIRT3 and PGC-1alpha in skeletal muscle of overweight adolescents without change in caloric intake. Pediatr Exerc Sci 27:177–184
Ward GR, Sutton JR, Jones NL, Toews CJ (1982) Activation by exercise of human skeletal muscle pyruvate dehydrogenase in vivo. Clin Sci (Lond) 63:87–92
Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL (2002) Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol 541:969–978
Watt MJ, Heigenhauser GJ, LeBlanc PJ, Inglis JG, Spriet LL, Peters SJ (2004) Rapid upregulation of pyruvate dehydrogenase kinase activity in human skeletal muscle during prolonged exercise. J Appl Physiol 97:1261–1267
White AT, Schenk S (2012) NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 303:E308–E321
Winder WW, Hardie DG (1996) Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Phys 270:E299–E304
Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B (1997) Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol 82:219–225
Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H (2002) Dissociation of AMPK activity and ACCbeta phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun 298:309–316
Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA (1999) Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 48:1593–1599
Acknowledgements
We would like to thank the subjects for an extraordinary effort, Professor Graham Hardie for providing anti-bodies, and, in regards to Peter Plomgaard, The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. The Centre of Inflammation and Metabolism (CIM) was supported by a grant from the Danish National Research Foundation (DNRF55) during the study period.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics Committee of Copenhagen and Frederiksberg communities (H-15010768) and was conducted in accordance with the guidelines of the Declaration of Helsinki. The subjects were informed about the experimental protocol, the risks, and discomforts that might occur in association with the intervention and provided written informed consent before the initiation of the study.
Funding
This study is funded by the Danish Ministry of Culture (1095421001), Danish Council for Independent Research (36723-104353), and LB: Danish Diabetes Academy (1105701001).
Rights and permissions
About this article
Cite this article
Gudiksen, A., Bertholdt, L., Stankiewicz, T. et al. Effects of training status on PDH regulation in human skeletal muscle during exercise. Pflugers Arch - Eur J Physiol 469, 1615–1630 (2017). https://doi.org/10.1007/s00424-017-2019-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2019-6