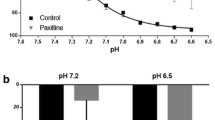
Abstract
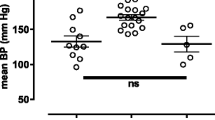
Changes in pH modulate the responsiveness of vascular smooth muscle cells to vasoconstrictor stimuli, but their effect on endothelium-dependent responses is unknown. Therefore, the influence of moderate changes in pH on responses to endothelium-dependent and –independent agonists was determined in aortae and carotid arteries of 15- to 26-week-old male C57BL/6N mice. Isolated rings were suspended in Halpern–Mulvany myographs for isometric tension recording. The preparations were exposed to either acidic (pH 7), control (pH 7.4) or alkaline (pH 7.8) modified Krebs–Ringer bicarbonate buffer solutions and their contractions and relaxations compared. Endothelium-dependent relaxations to acetylcholine (in the presence of meclofenamate or of the thromboxane-prostanoid (TP) receptor antagonist S18886) were comparable at the three pH values tested in contracted aortic or carotid arterial rings. Endothelium-dependent contractions of quiescent carotid arteries were reduced in acidosis and potentiated in alkalosis compared to control; these effects were reversible. The carotid arteries produced equal amounts of 6-keto prostaglandin F1α and thromboxane B2 at the different pH values tested. Contractions to the full TP receptor agonist U46619 were similar in the three milieus, but after inducing partial TP receptor blockade (with low concentrations of the TP receptor antagonist S18886) they were depressed in acidosis compared to alkalosis. Prostacyclin as a partial TP receptor activator also induced weaker contractions at low than at high pH, whereas its vasodilator effect was not affected. These findings demonstrate that changes in pH modulate endothelium-dependent contractions in mouse arteries primarily by altering the sensitivity of TP receptors of vascular smooth muscle to endothelium-derived contracting factors.








Similar content being viewed by others
References
Auch-Schwelk W, Katusic ZS, Vanhoutte PM (1992) Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension 19(5):442–445
Barton M, Baretella O, Meyer MR (2012) Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br J Pharmacol 165(3):591–602
Barton M, Haudenschild CC, d'Uscio LV, Shaw S, Münter K, Lüscher TF (1998) Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 95(24):14367–14372
Burget GE, Visscher MB (1927) Variations of the pH of the blood and the response of the vascular system to adrenalin. Am J Physiol 81(1):113–123
Campbell HDB, Crisp NW Jr, Weil MH, Brown EB Jr (1958) Depressed response to intravenous sympathicomimetic agents in humans during acidosis. Dis Chest 33(1):18–22
Chen G, Suzuki H, Weston AH (1988) Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 95(4):1165–1174
Christou H, Reslan OM, Mam V, Tanbe AF, Vitali SH, Touma M, Arons E, Mitsialis SA, Kourembanas S, Khalil RA (2012) Improved pulmonary vascular reactivity and decreased hypertrophic remodeling during nonhypercapnic acidosis in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302(9):L875–890
Crauwels HM, Van Hove CE, Herman AG, Bult H (2000) Heterogeneity in relaxation mechanisms in the carotid and the femoral artery of the mouse. Eur J Pharmacol 404(3):341–351
De Mey JG, Vanhoutte PM (1982) Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res 51(4):439–447
Félétou M, Vanhoutte PM (1988) Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol 93(3):515–524
Flavahan NA, Lindblad LE, Verbeuren TJ, Shepherd JT, Vanhoutte PM (1985) Cooling and alpha 1- and alpha 2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol 249(5 Pt 2):H950–955
Furchgott RF, Vanhoutte PM (1989) Endothelium-derived relaxing and contracting factors. FASEB J 3(9):2007–2018
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288(5789):373–376
Galla JH (2000) Metabolic alkalosis. J Am Soc Nephrol 11(2):369–375
Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM (1995) Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res 76(6):1003–1010
Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Félétou M (2005) Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol 146(6):834–845
Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC (1995) Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377(6546):239–242
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G (1987) Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84(24):9265–9269
Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, Symons JD, Richardson RS (2013) α1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Exp Physiol 98(1):256–267
Katusic ZS, Shepherd JT, Vanhoutte PM (1988) Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid, and acetylcholine in canine basilar arteries. Stroke 19(4):476–479
Li Z, Wang Y, Vanhoutte PM (2011) Upregulation of heme oxygenase 1 by hemin impairs endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Hypertension 58(5):926–934
Liu B, Luo W, Zhang Y, Li H, Zhu N, Huang D, Zhou Y (2012) Involvement of cyclo-oxygenase-1-mediated prostacyclin synthesis in the vasoconstrictor activity evoked by ACh in mouse arteries. Exp Physiol 97(2):277–289
Lüscher TF, Vanhoutte PM (1986) Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension 8(4):344–348
Meyer MR, Baretella O, Prossnitz ER, Barton M (2010) Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 86(1):58–64
Michel FS, Man RY, Vanhoutte PM (2007) Increased spontaneous tone in renal arteries of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 293(3):H1673–1681
Miller VM, Vanhoutte PM (1985) Endothelium-dependent contractions to arachidonic acid are mediated by products of cyclooxygenase. Am J Physiol 248(4 Pt 2):H432–437
Moncada S, Palmer RM, Higgs EA (1988) The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension 12(4):365–372
Mulvany MJ, Halpern W (1977) Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41(1):19–26
Nagao T, Vanhoutte PM (1991) Hyperpolarization contributes to endothelium-dependent relaxations to acetylcholine in femoral veins of rats. Am J Physiol 261(4 Pt 2):H1034–1037
Qu C, Leung SW, Vanhoutte PM, Man RY (2010) Chronic inhibition of nitric-oxide synthase potentiates endothelium-dependent contractions in the rat aorta by augmenting the expression of cyclooxygenase-2. J Pharmacol Exp Ther 334(2):373–380
Rapoport RM, Williams SP (1996) Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar–Kyoto rats. Hypertension 28(1):64–75
Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S (1990) Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101(3):746–752
Shepherd JT, Vanhoutte PM (1975) Veins and their control. W.B. Saunders, Philadelphia
Shepherd JT, Vanhoutte PM (1979) The human cardiovascular system: facts and concepts. Raven Press, New York
Shi Y, Ku DD, Man RY, Vanhoutte PM (2006) Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther 318(1):276–281
Simonet S, Descombes JJ, Vallez MO, Dubuffet T, Lavielle G, Verbeuren TJ (1997) S 18886, a new thromboxane (TP)-receptor antagonist is the active isomer of S 18204 in all species, except in the guinea-pig. Adv Exp Med Biol 433:173–176
Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G (2006) COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 291(3):H1429–1435
Tang EH, Félétou M, Huang Y, Man RY, Vanhoutte PM (2005) Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol 289(6):H2434–2440
Tang EH, Ku DD, Tipoe GL, Félétou M, Man RY, Vanhoutte PM (2005) Endothelium-dependent contractions occur in the aorta of wild-type and COX2−/− knockout but not COX1−/− knockout mice. J Cardiovasc Pharmacol 46(6):761–765
Tesfamariam B, Jakubowski JA, Cohen RA (1989) Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol 257(5 Pt 2):H1327–1333
Tobian L, Martin S, Eilers W (1959) Effect of pH on norepinephrine-induced contractions of isolated arterial smooth muscle. Am J Physiol 196(5):998–1002
Traupe T, Lang M, Goettsch W, Münter K, Morawietz H, Vetter W, Barton M (2002) Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens 20(11):2239–2245
Vanhoutte P, Clément D (1968) Effect of pH and PCO2 changes on the reactivity of isolated venous smooth muscle. Arch Int Physiol Biochim 76(1):144–146
Vanhoutte P, Leusen I (1967) Influence of variations of pH on the reactivity of isolated veins of the dog. C R Séances Soc Biol Fil 161(11):2318–2320
Vanhoutte P, Leusen I (1969) The reactivity of isolated venous preparations to electrical stimulation. Pflugers Arch 306(4):341–353
Vanhoutte PM, Shimokawa H, Tang EH, Félétou M (2009) Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196(2):193–222
Vanhoutte PM, Verbeuren TJ, Webb RC (1981) Local modulation of adrenergic neuroeffector interaction in the blood vessel wall. Physiol Rev 61(1):151–247
Verbeuren TJ, Janssens WJ, Vanhoutte PM (1978) Effects of moderate acidosis on adrenergic neurotransmission in canine saphenous veins. J Pharmacol Exp Ther 206(1):105–114
Yang D, Levens N, Zhang JN, Vanhoutte PM, Félétou M (2003) Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension 41(1):136–142
Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL (2005) Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol 289(3):H1027–1032
Acknowledgements
O.B. is grateful to Professor Matthias Barton for his long-time support and guidance. This study was supported by the Swiss National Science Foundation (grant PBZHP3-138754 to O.B.), the Hong Kong Research Grant Council (780410M), and by the World Class University program (R31-20029) funded by the Ministry of Education, Science and Technology, South Korea.
Disclosures
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baretella, O., Xu, A. & Vanhoutte, P.M. Acidosis prevents and alkalosis augments endothelium-dependent contractions in mouse arteries. Pflugers Arch - Eur J Physiol 466, 295–305 (2014). https://doi.org/10.1007/s00424-013-1323-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1323-z