Abstract
Using human H441 airway epithelial cells cultured at air–liquid interface (ALI), we have uniquely correlated the functional response to apical fluid volume expansion with the abundance and cleavage of endogenous α- and γENaC proteins in the apical membrane. Monolayers cultured at ALI rapidly elevated I sc when inserted into fluid-filled Ussing chambers. The increase in I sc was not significantly augmented by the apical addition of trypsin, and elevation was abolished by the protease inhibitor aprotinin and an inhibitor of the proprotein convertase, furin. These treatments also increased the IC50 amiloride indicating that the effect was via inhibition of highly Na+-selective ENaC channels. Apical fluid, 5–500 μl for 1 h in culture, increased the spontaneous starting I sc in a dose-dependent manner, whilst maximal fluid-induced I sc in the Ussing chamber was unchanged. Apical fluid expansion increased the abundance of 63–65-kDa αENaC proteins in the apical membrane. However, this could not be attributed to increased cleavage as protease inhibitors had no effect on the ratio of cleaved to non-cleaved (90 kDa) αENaC proteins. Instead, fluid expansion increased αENaC abundance in the membrane. In contrast, function correlated well with γENaC cleavage at known sites by furin and extracellular proteases. Interestingly, cleavage of γENaC was associated with increased retrieval from the membrane via the proteosomal pathway. Thus, the response to apical fluid volume expansion in H441 airway epithelial cells involves cleavage of γENaC, and changes in α- and γENaC protein abundance at the apical membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transepithelial transport of Na+ via amiloride-sensitive epithelial Na+ channels (ENaC) present in the apical membrane of airway epithelial cells regulates the depth of the fluid that lines the airways, airway surface liquid (ASL), which is critical for the function of the mucocilliary escalator and defence of the lung against infection. How a change in the depth of ASL is sensed and how ENaC activity is coordinately regulated are currently subject to intense scrutiny.
Protease cleavage of α- and γ ENaC protein subunits is associated with increased channel activity [1, 17–20, 35]. The Kunitz-type general serine protease inhibitor, aprotinin, inhibited ENaC activity in vitro and in vivo [7, 11, 32, 34] indicating that active serine proteases are present in ASL which increase the activity of ENaC and fluid absorption (for a review see [17]). A number of endogenous epithelial serine proteases have been identified in the airways and include the channel activating protein (CAP1) prostasin PRSS8 [13, 32, 40, 41], TMPRSS4 (CAP2), matriptase (CAP3) [29], and human neutrophil elastase [9]. ENaC activity is also regulated by the proprotein convertase, furin [18]. More recently, soluble protease inhibitors have also been proposed to be present in ASL such as α1-antitrypsin [27]. Their identification has driven the serine protease inhibitor balance hypothesis. In this model, elevation of ASL volume increases protease activity by diluting endogenous protease inhibitors which subsequently induces proteolytic activation of ENaC which drives ASL absorption. Conversely, dehydration of ASL concentrates protease inhibitors, reducing protease activity, proteolytic cleavage of ENaC and fluid absorption [23, 24, 31, 37].
Much of the work delineating which ENaC subunits are cleaved, where they are cleaved and how this affects the function of ENaC channels in the airway epithelial cell membrane has been addressed using epitope-tagged murine proteins expressed in Xenopus oocytes or heterologous expression systems. There is little information regarding the relationship between cleavage of endogenously expressed subunits and function, particularly in human airway epithelial cells.
Recently, Myerburg and colleagues, in a well-described functional study using human primary cultured airway epithelial cells, showed that increasing ASL volume activated ENaC activity [33]. Using pharmacological tools, they showed that this activation was in part due to protease activity but also appeared to involve brefeldin-sensitive trafficking of ENaC proteins to the apical membrane.
ENaC protease cleavage has not previously been investigated in a model of human Na+ absorptive airway epithelia (H441). Importantly, the effect of ASL volume expansion, exogenous protease and protease inhibition on amiloride-sensitive Na+ transport has not been correlated with the abundance and cleavage of endogenous α- and γENaC proteins expressed in the apical membrane of Na+ transporting cells. We now expand on previous observations and provide new and novel evidence that apical fluid expansion modifies cleavage of γENaC, and changes in α- and γENaC protein abundance at the apical membrane.
Materials and methods
Cell culture
H441 cells were purchased from American Type Culture Collection (Manassas, VA, USA). They were cultured in RPMI-1640 media supplemented with foetal bovine serum (10 %), l-glutamine (2 mM), sodium pyruvate (1 mM), insulin (5 μg ml−1), transferrin (5 μg ml−1), selenium selinite (7 ng ml−1) and antibiotics penicillin (100 U ml−1) and streptomycin (100 μg ml−1). Cells were seeded into 25-cm2 flasks and maintained in a humidified atmosphere with 5 % CO2 at 37°C.
Electrophysiology
Short circuit current (I sc) was measured across H441 cells that had been seeded onto Snapwell clear membranes (Corning, UK) and cultured overnight. When cells were fully confluent, the serum was replaced with 4% charcoal-stripped serum and supplementation of T3 (10 nM) and dexamethasone (200 nM). After 24 h, the apical media was removed, and the cells cultured for 7 days at air interface as previously described [6, 30, 42, 43]. Monolayers were deemed resistive if they exhibited a transepithelial electrical resistance (TEER) of approximately 200 Ω cm2. Monolayers were maintained at air–liquid interface (ALI) or exposed to apical fluid expansion with isotonic physiological salt solution (PSS) (5–500 μl) consisting of (in mM) NaCl 117, NaHCO3 25, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5 and d-glucose (11.0) (pH 7.4). Alternatively, monolayers were treated with vehicle, aprotinin (30 μM), dec-RVKR-cmk (40 μM, furin inhibitor, Calbiochem, UK) or trypsin (10 μM) in 100 μl PSS applied to the apical surface. All incubations were for 1 h. Monolayers were then mounted in Ussing chambers which contained PSS. This was maintained at 37°C and gassed with a premixed gas (21% O2 + 5% CO2). Spontaneous I sc was measured as the immediate I sc generated on introduction of the H441 monolayers to PSS in the Ussing chamber. Maximal I sc was measured at 5 min. To determine amiloride-sensitive I sc, amiloride (10−8–10−4 M) was added to the apical Ussing reservoir. Total current was determined by adding 1 mM ouabain to the basolateral reservoir. Monolayers were short circuited by clamping the potential difference at 0 mV using a DVC-4000 V/I clamp, and the current required to maintain this (I sc) was measured using a PowerLab computer interface. Every 30 s, a 2-mV pulse was applied to enable calculation of transepithelial resistance (TEER).
Cell surface biotinylation
Apical or basolateral membrane proteins were biotinylated as previously described [25, 42]. Briefly, after 7 days at air interface, polarised cells were washed with ice-cold PSS. Sulfo-NHS-biotin (0.5 mg/ml) was applied to the apical membrane and incubated for 30 min with gentle agitation. BSA (10%) in PSS was added to the basolateral bath to prevent biotinylation of basolateral proteins. Cells were then lysed, proteins solubilised and protein concentration was determined by Bradford assay. Similar amounts of total protein were incubated overnight with streptavidin agarose beads. The following day, biotinylated proteins bound to beads were separated from non-biotinylated proteins by centrifugation, washed and samples resuspended in 30 μl of protein loading buffer and prepared for immunoblotting.
Western blotting
Protein was separated by NuPAGE (Invitrogen) on 4–12% Bis–Tris gels and transferred to PVDF membrane. Membranes were blocked for 1 h at room temperature in 5 % w/v dried milk powder diluted in TBST (0.01% Tween-20 in Tris-Buffered Saline, pH 7.6). Primary antibodies were diluted in 1% w/v BSA in TBST. Membranes were incubated with primary antibodies overnight at 4°C, then washed three times for 5 min in TBST. Membranes were and incubated with secondary antibody diluted with 5% dried milk powder in TBST for 1 h at room temperature. Finally, membranes were washed three times for 5 min in TBST. Proteins were visualised using an enhanced chemiluminescence (ECL) Advance Detection Kit (Amersham Pharmacia Biotech, UK). Specificity of antibodies was confirmed by pre-incubation of antibody with excess antigenic peptide. Densitometry was performed on scanned blots using Scion Image Software (Scion Corp. USA).
Materials
Unless stated, all chemicals were obtained from Sigma Aldrich (Poole, UK). Primary antibodies were as follows: anti β-actin Abcam (Cambridge, UK); αENaC (designated RF in this study) was a kind gift of Prof. R. Frizzel, University of Pittsburgh School of Medicine, USA; α-ENaC C-terminal C20 and H95 antisera were purchased from Santa Cruz Biotechnology Inc. USA; and C-terminal γENaC from Sigma Aldrich, Poole, UK. HRP-conjugated secondary antibodies as appropriate were purchased from Upstate (Lake Placid, NY).
Statistical analyses
Statistical significance was determined using ANOVA with a post hoc paired or unpaired Student's t test where appropriate, and p values <0.05 were considered significant. All data are given as means ± SEM.
Results
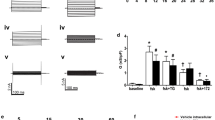
Spontaneous I sc was measured as the immediate I sc generated on introduction of the H441 monolayers to PSS in the Ussing chamber. Spontaneous I sc for cells cultured at air–liquid interface (ALI) was 10.2 ± 1.3 μA cm−2. I sc increased with a t 1/2 of 2.3 ± 0.1 min to reach a maximum of 22.8 ± 3 μA cm−2 at 5 min (p ≤ 0.001, n = 10; Figs. 1a and 3a). Application of amiloride (0.001–100 μM) inhibited most of the induced current. The maximum induced I amiloride was 19.0 ± 2.3 μA cm−2 (Figs. 1a and 3b). The calculated IC50 of the induced current was 0.3 μM (logEC50 −6.520 ± 0.087) (Fig. 4), and transepithelial resistance was 217 ± 21 Ω cm2.
Effect of apical fluid expansion and the presence and absence of trypsin on transepithelial I sc across H441 cells. Representative spontaneous short circuit current (I sc) traces from H441 monolayers after placement in Ussing chambers. a Cultured at air interface (ALI). b Cultured at ALI and exposed to trypsin in the Ussing chamber apical bath. c Cultured at ALI and exposed to 100 μl PSS on the apical surface for 1 h prior to placing in Ussing chamber. d Cultured at ALI and exposed to 100 μl PSS + trypsin on the apical surface for 1 h prior to placing in Ussing chamber. Once I sc had reached a stable level, amiloride (10−4–10−8 M) was applied to the apical chamber followed by ouabain (10−3 M) to the basolateral chamber
Effect of increased apical volume on the fluid-induced current
We investigated whether apical fluid expansion was responsible for the rise in I sc. Pre-incubation with 5–500 μl PSS applied to the apical surface for 1 h in culture increased the spontaneous I sc in a dose-dependent manner Fig. 5a. In samples pre-treated with 5–100 μl of apical fluid, I sc continued to rise to reach similar maximal levels at 5 min (p ≤ 0.05, n = 4). However, spontaneous I sc was similar to maximal I sc after pre-incubation with 500 μl apical fluid in culture (Fig. 5a).
Pre-treatment of cells with pharmacological agents required a minimum of 100 μl of PSS to be applied to the apical chamber. For comparative purposes, the spontaneous starting I sc for pre-treatment with 100 μl PSS was higher than monolayers maintained at ALI at 20.2 ± 3.2 μA cm−2 (p ≤ 0.01, n = 4). I sc increased to reach maximum values of 26.9 ± 3.8 μA cm−2 at 5 min (p < 0.05, n = 4) (Figs. 1c, 3a and 5a). Maximum I amiloride, IC50 amiloride and transepithelial resistance were similar to that of cells grown at ALI at 21.5 ± 2.5 μA cm−2, 0.3 μM (logEC50 −6.471 ± 0.15) and 212 ± 19 Ω cm2 (n = 4).
Upregulation of amiloride-sensitive I sc in H441 cells is associated with proteolytic activity
We reasoned that if upregulation of I sc was due to fluid activation of endogenous proteases, addition of trypsin to the apical chamber would augment the rise in I sc induced by apical fluid. This was not the case. Exposure to trypsin in the apical Ussing bath had no further effect on the spontaneous, maximal or time course of the induced I sc across cells grown at ALI (Fig. 1b). However, pre-treatment with apical trypsin for 1 h in culture increased the spontaneous starting I sc when cells were placed in the Ussing chamber and I sc did not rise. In contrast, I sc appeared to decrease. Mean values decreased from 26.1 ± 5 to 19.4 ± 4.7 μA cm−2 at 5 min although this did not quite reach significance (p = 0.18, n = 4) (Fig. 1d). The transepithelial resistance for monolayers treated with trypsin was not significantly different to those maintained at ALI. The calculated IC50 amiloride was 0.4 μM (logEC50 −6.402 ± 0.079) (Fig. 4).
We then inhibited protease activity with aprotinin and dec-RVKR-cmk peptide (a competitive furin inhibitor). The spontaneous I sc in aprotinin-treated cells was similar to that of cells grown at ALI at 8.3 ± 1.9 μA cm−2. Moreover, the I sc did not rise significantly (Fig. 2a). The maximum I sc at 5 min was 7.5 ± 1.8 μA cm−2 (n = 10, Figs. 1a, 2a and 3a). In the presence of aprotinin, maximal I amiloride was 4.9 ± 0.9 μA cm−2, significantly less than that of cells grown at ALI or incubated with 100 μl PSS alone (p < 0.01, n = 3 respectively). Notably, the TEER was reduced to 129 ± 31 Ω cm2 (p ≤ 0.05, n = 3), and the IC50 amiloride was significantly increased to 0.8 μM (logEC50 −6.11 ± 0.058) (p < 0.05, n = 3) (Figs. 2a, 3a, b and 4). In the presence of the furin inhibitor, the spontaneous starting I sc was 12.8 ± 3.5 μA cm−2, and there was a small apparent rise in I sc to 16.6 ± 3.8 μA cm−2 at 5 min, although this did not reach significance. The maximal I sc and I amiloride were both significantly less than that of monolayers grown at ALI (p < 0.05, n = 4) (Figs. 2b and 3a, b). The IC50 amiloride was significantly increased to 0.9 μM (logEC50 −6.027 ± 0.085) (p ≤ 0.05, n = 4) (Fig. 4). In the presence of furin ± aprotinin, starting I sc remained similar to that of furin alone at 15.5 ± 11.1 μA cm−2 and did not increase. I amiloride was 15.5 ± 5.0 μA cm−2 (n = 3). Transepithelial resistance was similar to that of aprotinin alone 138 ± 28 Ω cm2 (Figs. 2c and 3a, b).
Effect of protease inhibitors on transepithelial I sc across H441 cells. Representative spontaneous short circuit current (I sc) traces from H441 monolayers after placement in Ussing chambers. a Cultured at ALI and exposed to 100 μl PSS on the apical surface for 1 h in the presence of aprotinin. b Cultured at air interface and exposed to 100 μl PSS on the apical surface for 1 h in the presence of a furin inhibitor. c Cultured at air interface and exposed to 100 μl PSS on the apical surface for 1 h in the presence of aprotinin + furin inhibitor, all prior to placing in Ussing chamber. Once I sc had reached a stable level, amiloride (10−4–10−8 M) was applied to the apical chamber followed by ouabain (10−3 M) to the basolateral chamber
Apical fluid volume expansion increases spontaneous amiloride-sensitive I SC. a Starting spontaneous I sc (white bars) and maximum I sc (black bars) and b maximal amiloride-sensitive I sc extrapolated from traces for monolayers grown at air–liquid interface (ALI), exposed to 100 μl PSS on the apical surface for 1 h (100), exposed to 100 μl PSS on the apical surface for 1 h in the presence of aprotinin (A), exposed to 100 μl PSS on the apical surface for 1 h in the presence of the furin inhibitor and exposed to 100 μl PSS on the apical surface for 1 h in the presence of aprotinin and the furin inhibitor (A+F). Numbers of replicates for each treatment are given in “Results” section. Statistical significance was determined using paired or unpaired Student's t test as appropriate where *p < 0.05, **p < 0.01 and ***p < 0.001
Dose–response to amiloride in the presence and absence of protease and protease inhibitors. Amiloride (10−9–10−4 M) was added to the apical Ussing bath once maximal I sc had been established (see Fig. 1). Dose–response curves for amiloride are shown for monolayers grown at air–liquid interface (ALI), monolayers exposed to aprotinin (aprotinin), monolayers exposed to furin (furin) and monolayers exposed to trypsin (trypsin). The IC50 for aprotinin and furin inhibitor-treated cells, calculated from these curves, were significantly increased compared to cells grown at ALI (p ≤ 0.05, n = 3 and n = 4, respectively)
Fluid-induced amiloride-sensitive I sc is associated with α- and γENaC subunit proteolysis
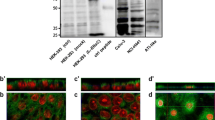
It is difficult to consistently identify endogenously expressed ENaC proteins human airway cells because cleavage affects which fragments contain the antigenic epitopes, and not all of these are known or published. Therefore, we used a number of different αENaC antisera to identify cleaved and uncleaved protein products in apical biotinylated protein extracts from H441 cells. The antiserum RF predominantly identified a 90-kDa protein in H441 cells that is consistent with the full-length αENaC protein and has been shown to identify similar protein in primary cultured human bronchiolar epithelial cell [28]. We also used a C-terminal antiserum C20, which faintly immunostained a protein of ∼130 kDa (consistent with the data sheet as full-length glycosylated ENaC) and predominantly recognised a protein of 65 kDa and a smaller product of 63 kDa in the apical membrane. We did not observe any additional products with the C20 antiserum which would be indicative of the proposed CAP protease cleavage of the C-terminal end of the ENaC protein. An antiserum directed against the N-terminal extracellular loop region also weakly immunostained a large protein of ∼100 kDa and two predominant products 65 and 63 kDa. A weaker protein was sometimes detected at 60 kDa. A diagram showing our suggested origin of these proteins is shown in Fig. 7. We used a γENaC antiserum directed against a C-terminal epitope (630–649) to investigate γENaC cleavage. This antiserum recognised a 90-kDa full-length protein and a smaller 75-kDa product consistent with N-terminal cleavage of the full-length protein (Fig. 7). There was also evidence of a further product of 74 kDa in most blots.
We firstly investigated whether the abundance of αENaC cleavage products were altered in response to apical fluid volume expansion. Using the C20 antiserum, we found that the abundance of the 65- and ∼63-kDa proteins increased in the apical membrane with increased fluid volume. These data indicated that raising the fluid volume increased the abundance of cleaved αENaC proteins in the apical membrane (Fig. 5b). To explore this further, we then investigated the abundance of full-length and cleaved αENaC proteins in monolayers that had been treated with 100 μl PSS, trypsin or protease inhibitors.
Effect of increasing apical fluid volume on spontaneous I sc and αENaC abundance in the apical membrane. a H441 monolayers were cultured at ALI or treated with 5–500-μl fluid on the apical surface for 1 h prior to mounting into Ussing chambers and recording I sc. Spontaneous starting I sc (white bars) and maximum I sc (black bars) are shown. Significantly different from starting I sc, *p < 0.05, **p < 0.01. Significantly different from starting I sc, †p < 0.05, ††p < 0.01. The number of replicates for each treatment is given in “Results” section. b Representative western blot of apical biotinylated protein from H441 monolayers cultured at ALI or treated with 50-, 100- or 500-μl fluid on the apical surface for 1 h. Blots were immunostained with antiserum C20 directed against αENaC that recognise 62–65-kDa proteins, respectively. c Densitometry analysis of proteins from blots as shown in b (n = 3). *p < 0.05, significantly different from ALI
Effect of apical fluid expansion on αENaC protein cleavage and abundance in the apical membrane of H441 cells. Western blot of apical biotinylated protein from H441 monolayers cultured at ALI or treated with 100 μl PSS alone (100) or 100 μl PSS containing aprotinin (A), furin inhibitor (F), aprotinin + furin inhibitor (A+F) or trypsin (T). a Representative immunoblot immunostained with RF antiserum that recognises a full-length 90-kDa αENaC protein with a graph below showing relative abundance of the 90-kDa ENaC protein analysed by densitometry and normalised to protein abundance in ALI samples. *p < 0.05 (n = 3), significantly different from ALI. b Representative immunoblot immunostained with C20 or H95 antiserum that recognise smaller cleaved proteins of 62–65 kDa with a graph below showing relative abundance of the predominant 65-kDa ENaC protein analysed by densitometry and normalised to protein abundance in ALI samples. *p < 0.05 (n = 7), significantly different from ALI
There was a significant increase in the abundance of the 90-kDa ENaC protein in monolayers treated with protease inhibitors (p < 0.05, n = 3). The abundance of protein in samples treated with 100 μl PSS was variable, and monolayers treated with trypsin were similar to that of cells maintained at ALI (Fig. 6a). We anticipated that there would be a change in abundance of the smaller protein products recognised by our antisera consistent with cleavage or inhibition of cleavage of αENaC protein. However, this was not the case. Similar to that observed with the full-length protein, using two independent antisera (H95 and C20), we found that apical protein abundance increased in all monolayers except those treated with trypsin (p < 0.05, n = 7) (Fig. 6b). The decreased abundance of protein in the trypsin-treated samples could not be attributed to a simple loss of epitopes by cleavage because the three antisera target different parts of the protein and there was no change in the sizes of the cleaved products (Fig. 7). We could not detect any significant change in the ratio of abundance of the 90:65-kDa proteins in the aprotinin- or furin-treated monolayers that would demonstrate inhibition of cleavage. Moreover, although the ratio of the 90:65-kDa proteins appeared to decrease in some samples from monolayers that were treated with 100 μl of PSS alone or trypsin (indicating increased cleavage), these findings were not consistent. Thus, our data support the notion that apical fluid and/or protease inhibitors increased the abundance of αENaC proteins in the apical membrane and that the presence of trypsin modified this process.
Schematic diagram of proposed cleavage products identified with α- and γENaC antisera. Proposed sizes of non-cleaved and protease cleaved protein products identified by RF, H95 and C20 antisera in human αENaC and by the C-terminal antiserum in human γENaC. Main conserved cleavage sites were identified using sequence comparison and published sites from murine sequences [15, 26, 35]
We then investigated the abundance and cleavage of γENaC proteins in the apical membrane of H441 cell monolayers. There was a decrease in total apical abundance of γENaC protein in monolayers exposed to 100 μl of apical fluid alone or with trypsin (p < 0.05, n = 3) (Fig. 8b). The absolute abundance of the full-length 90-kDa and cleaved 75-kDa proteins varied between batches of monolayers. However, the ratio of 90:75-kDa protein revealed that the cleaved to uncleaved protein was increased in monolayers exposed to 100-μl apical fluid alone or trypsin (p < 0.05, n = 3) (Fig. 8c). In monolayers treated with protease inhibitors, the cleaved to non-cleaved protein was markedly reduced (p < 0.05, n = 3). Whilst we observed a smaller fainter cleavage product of 74-kDa product in most of our blots, we could not discern any treatments which specifically altered the abundance of this product. Comparing the cleaved to non-cleaved protein ratio with spontaneous I sc values revealed that there was a significant correlation with function (r 2 value = 0.84, p ≤ 0.01) (Fig. 8d). These data indicate that γENaC cleavage is an important factor mediating channel activity in H441 cells.
Effect of protease activity and apical fluid expansion on γENaC protein cleavage in the apical membrane of H441 cells. Western blot of apical biotinylated protein from H441 monolayers cultured at ALI or treated with 100 μl PSS alone (100) or 100 μl PSS containing aprotinin (A), furin inhibitor (F), aprotinin + furin inhibitor (A+F) or trypsin (T). a Representative immunoblot immunostained with a C-terminal antiserum that recognises a full-length 90 kDa and a predominant 75-kDa cleaved γENaC protein. b Graph of relative abundance of the cleaved/non-cleaved γENaC proteins analysed by densitometry. *p < 0.05 (n = 3), statistically different from ALI. C. Graph of relative abundance of the cleaved + non-cleaved γENaC proteins analysed by densitometry. *p < 0.05 (n = 3), statistically different from ALI. c Graph of cleaved/non-cleaved γENaC proteins values plotted against spontaneous starting I sc (μA cm−2) (see Fig. 3a). The correlation value of the line (r 2) is shown in the top right of the graph
Because total γENaC protein abundance decreased with cleavage, we further investigated this phenomenon. We tested the hypothesis that protease cleavage promoted protein retrieval from the membrane. Inhibition of the proteosomal retrieval pathway by MG132 increases I sc and γENaC abundance in the membrane of H441 cells [2]. Pre-treatment of cells with MG132 in the basolateral chamber prevented the trypsin-induced decrease in apical abundance of γENaC (Fig. 9a).
Effect of the proteosomal inhibitor MG132 on γENaC protein abundance in the apical membrane of H441 cells. Western blot of apical biotinylated protein from H441 monolayers cultured at ALI or treated with 100 μl PSS + trypsin (T) in the presence and absence of the proteasome inhibitor MG132 immunostained with a C-terminal antiserum that recognises a full-length 90- and 75-kDa cleaved γENaC proteins. This blot was repeated with similar results
Discussion
These studies provide a new and novel perspective on ENaC cleavage in human airway. There is a large body of evidence showing that fluid-induced proteolytic cleavage of ENaC increases its activity in the lung [16, 20, 32, 33, 35]. However, it is important to point out that most of the information regarding protease cleavage sites and their functional consequence has been obtained from epitope-tagged murine ENaC proteins expressed in Xenopus oocytes or heterologous expression systems. In this study, we have critically examined function together with the abundance and cleavage of endogenous α- and γENaC in polarised human airway epithelial cells [17, 18].
H441 cells grown at ALI and then exposed to apical fluid expansion in the Ussing chamber system rapidly increased I sc in a manner similar to that described by Myerburg et al. in primary cultured human bronchiolar epithelial cells (HBEC) [32, 33]. We also demonstrated that there was a graded response in I sc to apical fluid expansion over a range of 5–500 μl (within 1 h in culture) indicating that this response was volume dependent. In the Ussing chamber, maximal currents were observed approximately 5 min after volume expansion, an effect more rapid than that shown in HBECs. Mechanical forces generated by changes in fluid volume (pressure) and/or flow (shear stress) are known to alter amiloride-sensitive I sc and increase the open probability of channels in the membrane [3, 36, 39]. The response to shear stress of human αβγENaC expressed in Xenopus oocytes was also augmented by protease cleavage [14]. Thus, the very rapid elevation of I sc in our Ussing chambers could be the result of both volume expansion and mechanical shear forces elicited by the circulating fluid.
ASL fluid height is estimated to be approximately 7 μm in ciliated airway cells grown at ALI [38]. Thus, 5 μl applied to the surface of these cells represents an increase to approximately 50-μm depth of fluid overlying the cells (although the formation of a meniscus in the Transwell support means that the actual depth is likely to be lower). In H441 cells, a 50% increase in I sc at 1 h occurred at a fluid depth of approximately 650 μm, over 100× that of the predicted starting ASL height and a significant expansion of ASL volume. Over such a volume range, we question whether the rise in I sc can be solely due to dilution of protease inhibitors and cleavage of existing protein in the H441 cells apical membrane. We would like to suggest that there may also be a role for increased turnover of channels in the membrane as apical fluid expansion increases.
Both aprotinin and the furin inhibitor prevented the fluid-induced rise in I sc, consistent with an action of both extracellular serine protease(s) and furin on amiloride-sensitive Na± channels. H441 cells express two populations of amiloride-sensitive channels in the apical membrane [2]. The rightward shift in the IC50 amiloride would indicate that the predominant effect of protease inhibitors was on highly Na±-selective, low-conductance amiloride-sensitive ENaC channels. These data were consistent with findings in HBECs [33]. However, the decreased TEER of H441 monolayers in the presence of aprotinin could provoke indirect effects on I sc. We therefore interpret these data with caution. Moreover, we speculate that the reduction in TEER may implicate a role for serine proteases in barrier formation in these cells [8].
In the presence of the furin inhibitor, there was only a small rise in I sc in the Ussing chamber indicating that serine proteases alone had a minor effect on amiloride-sensitive Na+ channel function. The inhibitory effect of both protease inhibitors was not additive indicating that both proteases were required for maximal channel activity. A number of protease cleavage sites have been identified in murine α- and γENaC subunits of ENaC that are conserved in the human sequence and modulate channel activity [1, 15, 17, 18, 35]. Furin cleaved mouse αENaC at two independent sites in the N-terminal in heterologous expression systems [18]. Potential CAP cleavage sites were also present in the N-terminal and C-terminal region of αENaC. In the apical membrane of H441 cells, we observed proteins of 90 kDa consistent with the full-length protein and 63–65 kDa which were indicative of N-terminal cleavage of human αENaC protein at similar furin/CAP cleavage sites [5, 21, 28]. In addition, cleavage of one or both sites would result in the generation of proteins that differ in size by approximately 2.5 kDa. Thus, the 65- and 63-kDa products could potentially be the result of cleavage at one or both sites, respectively. We did not observe any smaller products with the C20 antiserum that would indicate CAP protease cleavage of the C-terminal end of the ENaC protein.
Surprisingly, however, inhibition of proteases with aprotinin and/or the furin inhibitor did not affect the sizes of the observed αENaC protein products. Also, we could not determine a measureable change in the ratio of 90- (full length), 65- or 63-kDa proteins that would indicate alterations or inhibition of protease cleavage with any of the treatments. It is hard to explain these findings. It is obviously more difficult to compare endogenous cleavage products using available αENaC antisera compared to epitope-tagged proteins in over-expression systems. Differing epitopes and antibody affinities certainly make it more difficult to correlate cleaved and non-cleaved products by western blotting. Because of this, it may not be viable to detect subtle changes in αENaC cleavage particularly when overall abundance was also seen to change. Alternatively, it is possible that endogenous αENaC proteins in H441 cells are subject to different, cell-specific protease cleavage that is aprotinin and dec-RVKR insensitive. For example, α1-antitrypsin decreases I Na+ in H441 cells grown on coverslips and inhibits the activity of the protease matriptase/CAP-3 which is aprotinin insensitive [22, 27]. CAP3 has not yet been shown to cleave αENaC directly, but co-expression of mouse CAP3 with ENaC subunits in Xenopus oocytes increased channel activity [4]. Given that cleavage was observed but did not alter in all conditions tested, we can only conclude at this point that increased αENaC cleavage did not mediate fluid-induced I sc in H441 cells.
In contrast, treatment with aprotinin and the furin inhibitor did alter the cleaved (75 kDa) to uncleaved (90 kDa) ratio of γENaC indicating that reduced cleavage of γENaC was associated with the reduction of I sc measured across these cells. The ratio of cleaved to uncleaved γENaC correlated very closely with function indicating that γENaC cleavage was an important factor in fluid-induced channel activity and amiloride-sensitive I sc in H441 cells. Cleavage of rat γENaC protein has been shown to elevate channel function in Xenopus oocytes [12]. However, this is the first demonstration of changes in abundance of cleaved and non-cleaved endogenous γENaC protein in the apical membrane of human airway epithelial cells and its relationship to functional amiloride-sensitive Na+ transport.
γENaC is cleaved by furin and CAP-2 at a site in the N-terminal region R138–R186 of the protein. This region forms an inhibitory domain, and its cleavage increased ENaC activity [10, 12, 15]. γENaC also contains consensus sites for a number of other proteases including prostasin, elastase and plasmin (see [20] for review) which can activate channel activity without affecting αENaC cleavage. Our finding that the furin inhibitor and aprotinin were not functionally additive would support a requirement for cleavage by both proteases, at similar residues, consistent with the findings of Garcia-Caballero et al. [15]. Further work will be required to identify whether the extracellular protease responsible is CAP-2 in H441 cells.
Two other novel observations arose from these studies. First was that the abundance of cleaved and non-cleaved αENaC proteins increased in the apical membrane when the fluid volume was raised, particularly at high apical volume (500 μl), when treated with protease inhibitors, and not when treated with trypsin. Thus, we suggest that there is increased translocation of αENaC to the apical membrane in response to apical fluid volume expansion, particularly when channel function is reduced, as an additional/alternative mechanism to regulate airway fluid volume. This is supported by recent studies in HBECs, which showed that part of the rise in I sc in response to increased apical fluid volume was prevented by pharmacological inhibitors of protein trafficking [33]. Second, we showed that total γENaC protein decreased in the apical membrane when proteolytically cleaved by endogenous proteins and trypsin and that this was likely via increased retrieval and proteosomal degradation. In oocytes, αENaC was effectively translocated to the membrane whether in a cleaved or non-cleaved form but β and γ did not traffic independently [17]. Moreover, these authors also showed that αENaC and γENaC were only cleaved when expressed together. Thus, we propose that increased apical fluid volume activated proteases that cleave γENaC. This process also promoted retrieval. In the face of increased fluid volume, additional new subunits must therefore be inserted into the apical membrane to maintain transport. This would require increased translocation of αENaC with γENaC to the membrane. As translocation may also involve αENaC alone or αENaC associated with other subunits could explain the difference in observed apical abundance of the two subunits. Thus, a balance between cleavage and subunit turnover is required to retain sufficient active channels in the membrane to maintain effective transport processes and resolve apical fluid excess in the airway. In this regard, it is worth noting that pre-treatment with exogenous trypsin decreased both α- and γENaC abundance and led to more rapid rundown of I sc in the Ussing chamber. In this situation, perhaps the balance is pushed more to retrieval than insertion. In the light of these findings, new questions now arise. First, why and how does cleavage promote ENaC retrieval in these cells? Second, what are the signalling mechanisms that promote translocation of ENaC channels to the membrane? One possibility, suggested by Myerburg and colleagues, is a change in ASL osmolarity during apical fluid expansion. This may have effects on cell volume which have been shown by us and others to affect αENaC translocation [33, 42]. Further work is now required to answer these questions.
In conclusion, we have provided new evidence to show that cleavage of endogenous γENaC protein is associated with increased ENaC activity in response to apical fluid volume expansion in H441 airway epithelial cells monolayers. However, cleavage of γENaC also appears to decrease its abundance in the membrane via proteosomal processing. In the presence of protease inhibitors, apical fluid volume expansion increased the abundance of αENaC and maintained the abundance of γENaC proteins in the apical membrane indicating increased trafficking of ENaC subunits. Thus, increased turnover of ENaC proteins may provide an additional mechanism to increase the number of active channels in the apical membrane to help maintain correct ASL volume.
References
Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ (2007) A segment of gamma ENaC mediates elastase activation of Na + transport. J Gen Physiol 130(6):611–629. doi:10.1085/jgp.200709781
Albert AP, Woollhead AM, Mace OJ, Baines DL (2008) AICAR decreases the activity of two distinct amiloride-sensitive Na + −permeable channels in H441 human lung epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 295(5):L837–L848
Althaus M, Bogdan R, Clauss WG, Fronius M (2007) Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21(10):2389–2399. doi:10.1096/fj.06-7694com
Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC (2006) Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol 17(4):968–976
Baines DL, Albert AP, Hazell MJ, Gambling L, Woollhead AM, Dockrell ME (2010) Lipopolysaccharide modifies amiloride-sensitive Na + transport processes across human airway cells: role of mitogen-activated protein kinases ERK 1/2 and 5. Pflugers Arch 459(3):451–463. doi:10.1007/s00424-009-0717-4
Baloglu E, Ke A, Abu-Taha IH, Bartsch P, Mairbaurl H (2009) In vitro hypoxia impairs beta2-adrenergic receptor signaling in primary rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 296(3):L500–L509. doi:10.1152/ajplung.90390.2008
Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR (2007) Epithelial Na + channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282(9):6153–6160. doi:10.1074/jbc.M610636200
Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM (2010) Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A 107(9):4200–4205. doi:10.1073/pnas.0903923107
Caldwell RA, Boucher RC, Stutts MJ (2004) Serine protease activation of near-silent epithelial Na + channels. Am J Physiol Cell Physiol 286(1):C190–C194
Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR (2008) Defining an inhibitory domain in the alpha-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 294(1):F47–F52
Coote KJ, Atherton H, Young A, Sugar R, Burrows R, Smith NJ, Schlaeppi JM, Groot-Kormelink PJ, Gosling M, Danahay H (2008) The guinea-pig tracheal potential difference as an in vivo model for the study of epithelial sodium channel function in the airways. Br J Pharmacol 155(7):1025–1033. doi:10.1038/bjp.2008.363
Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C (2008) Cleavage in the {gamma}-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol 586(Pt 19):4587–4608. doi:10.1113/jphysiol.2008.154435
Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE (2002) Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem 277(10):8338–8345. doi:10.1074/jbc.M105044200
Fronius M, Bogdan R, Althaus M, Morty RE, Clauss WG (2010) Epithelial Na + channels derived from human lung are activated by shear force. Respir Physiol Neurobiol 170(1):113–119. doi:10.1016/j.resp.2009.11.004
Garcia-Caballero A, Dang Y, He H, Stutts MJ (2008) ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol 132(5):521–535. doi:10.1085/jgp.200810030
Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC (2007) A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 282(1):58–64. doi:10.1074/jbc.M605125200
Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC (2008) Preferential assembly of epithelial sodium channel (ENaC) subunits in Xenopus oocytes: role of furin-mediated endogenous proteolysis. J Biol Chem 283(12):7455–7463. doi:10.1074/jbc.M707399200
Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR (2004) Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279(18):18111–18114
Hughey RP, Bruns JB, Kinlough CL, Kleyman TR (2004) Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279(47):48491–48494
Hughey RP, Carattino MD, Kleyman TR (2007) Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens 16(5):444–450
Itani OA, Auerbach SD, Husted RF, Volk KA, Ageloff S, Knepper MA, Stokes JB, Thomas CP (2002) Glucocorticoid-stimulated lung epithelial Na(+) transport is associated with regulated ENaC and sgk1 expression. Am J Physiol Lung Cell Mol Physiol 282(4):L631–L641
Janciauskiene S, Nita I, Subramaniyam D, Li Q, Lancaster JR Jr, Matalon S (2008) Alpha1-antitrypsin inhibits the activity of the matriptase catalytic domain in vitro. Am J Respir Cell Mol Biol 39(6):631–637. doi:10.1165/rcmb.2008-0015RC
Joo NS, Irokawa T, Robbins RC, Wine JJ (2006) Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem 281(11):7392–7398. doi:10.1074/jbc.M512766200
Joo NS, Lee DJ, Winges KM, Rustagi A, Wine JJ (2004) Regulation of antiprotease and antimicrobial protein secretion by airway submucosal gland serous cells. J Biol Chem 279(37):38854–38860. doi:10.1074/jbc.M407077200
Kalsi KK, Baker EH, Medina RA, Rice S, Wood DM, Ratoff JC, Philips BJ, Baines DL (2008) Apical and basolateral localisation of GLUT2 transporters in human lung epithelial cells. Pflugers Arch 456(5):991–1003
Kleyman TR, Carattino MD, Hughey RP (2009) ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284(31):20447–20451. doi:10.1074/jbc.R800083200
Lazrak A, Nita I, Subramaniyam D, Wei S, Song W, Ji HL, Janciauskiene S, Matalon S (2009) Alpha(1)-antitrypsin inhibits epithelial Na + transport in vitro and in vivo. Am J Respir Cell Mol Biol 41(3):261–270. doi:10.1165/rcmb.2008-0384OC
Liang X, Butterworth MB, Peters KW, Frizzell RA (2010) AS160 modulates aldosterone-stimulated epithelial sodium channel forward trafficking. Mol Biol Cell 21(12):2024–2033. doi:10.1091/mbc.E10-01-0042
List K, Hobson JP, Molinolo A, Bugge TH (2007) Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol 213(1):237–245. doi:10.1002/jcp.21115
Mace OJ, Woollhead AM, Baines DL (2008) AICAR activates AMPK and alters PIP2 association with the epithelial sodium channel ENaC to inhibit Na + transport in H441 lung epithelial cells. J Physiol 586(Pt 18):4541–4557. doi:10.1113/jphysiol.2008.158253
Marlor CW, Delaria KA, Davis G, Muller DK, Greve JM, Tamburini PP (1997) Identification and cloning of human placental bikunin, a novel serine protease inhibitor containing two Kunitz domains. J Biol Chem 272(18):12202–12208
Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM (2006) Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem 281(38):27942–27949
Myerburg MM, Harvey PR, Heidrich EM, Pilewski JM, Butterworth MB (2010) Acute regulation of the epithelial sodium channel in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol 43(6):712–719. doi:10.1165/rcmb.2009-0348OC
Planes C, Leyvraz C, Uchida T, Angelova MA, Vuagniaux G, Hummler E, Matthay M, Clerici C, Rossier B (2005) In vitro and in vivo regulation of transepithelial lung alveolar sodium transport by serine proteases. Am J Physiol Lung Cell Mol Physiol 288(6):L1099–L1109
Rossier BC, Stutts MJ (2009) Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71:361–379
Satlin LM, Sheng S, Woda CB, Kleyman TR (2001) Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol 280(6):F1010–F1018
Tarran R (2004) Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc 1(1):42–46. doi:10.1513/pats.2306014
Tarran R (2008) Raising the volume on near-silent epithelial Na + channels. J Physiol 586(Pt 19):4583–4584
Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC (2005) Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280(42):35751–35759. doi:10.1074/jbc.M505832200
Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH (2004) Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 287(5):L928–L935
Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC (1997) An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389(6651):607–610. doi:10.1038/39329
Woollhead AM, Baines DL (2006) Forskolin-induced cell shrinkage and apical translocation of functional EGFP-human alpha ENaC in H441 lung epithelial cell monolayers. J Biol Chem 281(8):5158–5168
Woollhead AM, Scott JW, Hardie DG, Baines DL (2005) Phenformin and 5-aminoimidazole-4-carboxamide-1-{beta}-D-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na + transport across H441 lung cells. J Physiol 566(Pt 3):781–792
Acknowledgements
We would like to thank Prof R Frizzell for his kind gift of αENaC antiserum. This work was funded in part by Biotechnology and Biological Sciences Research Council Project Grant BB/E013597/1, Wellcome Trust Project Grant PG/07/079/23568 and St George’s University of London (to D.L.B.)
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
A. Representative western blot of apical biotinylated or non-bound protein from H441 monolayers cultured at ALI or treated with 100 μl PSS alone (100) or 100 μl PSS containing aprotinin (A), furin inhibitor (F), aprotinin + furin inhibitor (A + F) or trypsin (T) and immunostained with antiserum directed against β-actin. B. Western blots of H441 protein lysate (L) or apical biotinylated protein (A) immunostained with H95, C20 or RF antisera in the presence and absence of immunogenic peptide (+P) (not available for RF antiserum) (TIF 208 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tan, C.D., Selvanathar, I.A. & Baines, D.L. Cleavage of endogenous γENaC and elevated abundance of αENaC are associated with increased Na+ transport in response to apical fluid volume expansion in human H441 airway epithelial cells. Pflugers Arch - Eur J Physiol 462, 431–441 (2011). https://doi.org/10.1007/s00424-011-0982-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0982-x