Abstract
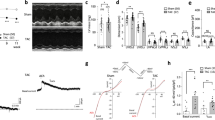
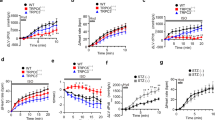
The α1-adrenoceptor as well as the AT1- and the ETA-receptor couple to G-proteins of the Gαq/11 family and contribute to the regulation of the transient outward K+ current (Ito,f) under pathological conditions such as cardiac hypertrophy or failure. This suggests an important role of Gαq/11-signalling in the physiological regulation of Ito,f. Here, we investigate mice deficient of the Gα11 protein (gna11−/−) to clarify the physiological role of Gα11 signalling in cardiac ion channel regulation. Myocytes from endocardial and epicardial layers were isolated from the left ventricular free wall and investigated using the ruptured-patch whole-cell patch-clamp technique. At +40 mV, epicardial myocytes from gna11−/− mice displayed a 23% larger Ito,f than controls (52.6 ± 4.1 pApF−1, n = 20 vs 42.7 ± 2.8 pApF−1, n = 26, p < 0.05). Endocardial Ito,f was similar in gna11−/− mice and controls. With the except of minor changes in endocardial myocytes, Ito,f kinetics were similar in both groups. In the epicardial layer, western blot analysis revealed a 19% higher expression of the K+-channel α-subunit Kv4.2 in gna11−/− mice than in wild type (wt; p < 0.05). The β-subunit KChIP2b was upregulated by 102% in epicardial myocytes of gna11−/− mice (p < 0.01, n = 4). Consistent with the difference in Ito,f, action potential duration was shorter in epicardial cells of gna11−/− mice than in wt (p < 0.05), while no difference was found in endocardial myocytes. These results suggest that Gα11-coupled signalling is a central pathway in the regulation of Ito,f. It physiologically exerts a tonic inhibitory influence on the expression of Ito,f and thereby contributes to the regulation of cardiac repolarisation.





Similar content being viewed by others
References
Agus ZS, Dukes ID, Morad M (1991) Divalent cations modulate the transient outward current in rat ventricular myocytes. Am J Physiol 261:C310–C318
Benitah JP, Vassort G (1999) Aldosterone upregulates Ca2+ current in adult rat cardiomyocytes. Circ Res 85:1139–1145
Brouillette J, Clark RB, Giles WR, Fiset C (2004) Functional properties of K+ currents in adult mouse ventricular myocytes. J Physiol 559:777–798
Brunet S, Aimond F, Guo W, Li H, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM (2004) Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559:103–120
Clark RB, Bouchard RA, Salinas SE, Sanchez-Chapula JA, Giles WR (1993) Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res 27:1795–1799
Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, Zhu W, Lebel M, Cheng CW, Park CY, Pierce SA, Guerchicoff A, Pollevick GD, Chan TY, Kabir MG, Cheng SH, Husain M, Antzelevitch C, Srivastava D, Gross GJ, Hui CC, Backx PH, Bruneau BG (2005) The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell 123:347–358
Goldsmith ZG, Dhanasekaran DN (2007) G protein regulation of MAPK networks. Oncogene 26:3122–3142
Goltz D, Schultz JH, Stucke C, Wagner M, Bassalay P, Schwoerer AP, Ehmke H, Volk T (2007) Diminished Kv4.2/3 but not KChIP2 levels reduce the cardiac transient outward K+ current in spontaneously hypertensive rats. Cardiovasc Res 74:85–95
Gotoh Y, Imaizumi Y, Watanabe M, Shibata EF, Clark RB, Giles WR (1991) Inhibition of transient outward K+ current by DHP Ca2+ antagonists and agonists in rabbit cardiac myocytes. Am J Physiol 260:H1737–H1742
Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM (2005) Targeted deletion of Kv4.2 eliminates Ito,f and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res 97:1342–1350
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100
Isenberg G, Klöckner U (1982) Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium”. Pflugers Arch 395:6–18
Jia Y, Takimoto K (2006) Mitogen-activated protein kinases control cardiac KChIP2 gene expression. Circ Res 98:386–393
Jiang M, Xu X, Wang Y, Toyoda F, Liu XS, Zhang M, Robinson RB, Tseng GN (2009) Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2. J Biol Chem 284:16452–16462
Kamp TJ, Hell JW (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87:1095–1102
Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyen-Tran VT, Gu Y, Ikeda Y, Chu PH, Ross J, Giles WR, Chien KR (2001) A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of Ito and confers susceptibility to ventricular tachycardia. Cell 107:801–813
Li J, Marionneau C, Zhang R, Shah V, Hell JW, Nerbonne JM, Anderson ME (2006) Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ Res 99:1092–1099
Litovsky SH, Antzelevitch C (1988) Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res 62:116–126
Matsumoto Y, Aihara H, Yamauchi-Kohno R, Reien Y, Ogura T, Yabana H, Masuda Y, Sato T, Komuro I, Nakaya H (2002) Long-term endothelin A receptor blockade inhibits electrical remodeling in cardiomyopathic hamsters. Circulation 106:613–619
Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456
Nerbonne JM, Kass RS (2005) Molecular physiology of cardiac repolarization. Physiol Rev 85:1205–1253
Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM (2008) Kv4.3 is not required for the generation of functional Ito,f channels in adult mouse ventricles. J Mol Cell Cardiol 44:95–104
Offermanns S, Zhao LP, Gohla A, Sarosi I, Simon MI, Wilkie TM (1998) Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/Gα11-mutant mice. EMBO J 17:4304–4312
Perrier E, Perrier R, Richard S, Benitah JP (2004) Ca2+ controls functional expression of the cardiac K+ transient outward current via the calcineurin pathway. J Biol Chem 279:40634–40639
Pourrier M, Schram G, Nattel S (2003) Properties, expression and potential roles of cardiac K+ channel accessory subunits: MinK, MiRPs, KChIP, and KChAP. J Membr Biol 194:141–152
Rivard K, Paradis P, Nemer M, Fiset C (2008) Cardiac-specific overexpression of the human type 1 angiotensin II receptor causes delayed repolarization. Cardiovasc Res 78:53–62
Rossow CF, Dilly KW, Santana LF (2006) Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ Res 98:1306–1313
Rossow CF, Dilly KW, Yuan C, Nieves-Cintron M, Cabarrus JL, Santana LF (2009) NFATc3-dependent loss of Ito gradient across the left ventricular wall during chronic beta adrenergic stimulation. J Mol Cell Cardiol 46:249–256
Rossow CF, Minami E, Chase EG, Murry CE, Santana LF (2004) NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circ Res 94:1340–1350
Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH (2003) Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J Physiol 546:5–18
Suzuki T, Takimoto K (2005) Differential expression of Kv4 pore-forming and KChIP auxiliary subunits in rat uterus during pregnancy. Am J Physiol Endocrinol Metab 288:E335–E341
Teutsch C, Kondo RP, Dederko DA, Chrast J, Chien KR, Giles WR (2007) Spatial distributions of Kv4 channels and KChip2 isoforms in the murine heart based on laser capture microdissection. Cardiovasc Res 73:739–749
Tomaselli GF, Zipes DP (2004) What causes sudden death in heart failure? Circ Res 95:754–763
van der Heyden MA, Wijnhoven TJ, Opthof T (2006) Molecular aspects of adrenergic modulation of the transient outward current. Cardiovasc Res 71:430–442
Volk T, Ehmke H (2002) Conservation of L-type Ca2+ current characteristics in endo- and epicardial myocytes from rat left ventricle with pressure-induced hypertrophy. Pflugers Arch 443:399–404
Volk T, Nguyen THD, Schultz JH, Faulhaber J, Ehmke H (2001) Regional alterations of repolarizing K+ currents among the left ventricular wall of rats with ascending aortic stenosis. J Physiol 530:443–455
Wagner M, Goltz D, Stucke C, Schwoerer AP, Ehmke H, Volk T (2007) Modulation of the transient outward K+ current by inhibition of endothelin-A receptors in normal and hypertrophied rat hearts. Pflugers Arch 454:595–604
Wettschureck N, Offermanns S (2005) Mammalian G proteins and their cell type specific functions. Physiol Rev 85:1159–1204
Wettschureck N, Rutten H, Zywietz A, Gehring D, Wilkie TM, Chen J, Chien KR, Offermanns S (2001) Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat Med 7:1236–1240
Wettwer E, Amos GJ, Posival H, Ravens U (1994) Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res 75:473–482
Xu H, Guo W, Nerbonne JM (1999) Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol 113:661–678
Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS (2000) Effects of the renin-angiotensin system on the current Ito in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res 86:1062–1068
Acknowledgments
We gratefully acknowledge the expert technical assistance of Céline Harlay. We are indebted to Dr. Thomas Wilkie, UT Southwestern, Dallas, TX, USA, for generating and to Dr. Nina Wettschureck and Dr. Stefan Offermanns, Max-Planck-Institut für Herz- und Lungenforschung, Bad Nauheim, Germany, for providing the gna11−/− mice.
Sources of funding
This work is supported by the Deutsche Forschungsgemeinschaft.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Michael Wagner and Elena Rudakova contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wagner, M., Rudakova, E., Schütz, V. et al. Larger transient outward K+ current and shorter action potential duration in Gα11 mutant mice. Pflugers Arch - Eur J Physiol 459, 607–618 (2010). https://doi.org/10.1007/s00424-009-0762-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0762-z