Abstract
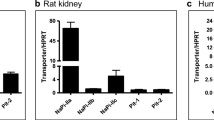
During metabolic acidosis (MA), urinary phosphate excretion increases and contributes to acid removal. Two Na+-dependent phosphate transporters, NaPi-IIa (Slc34a1) and NaPi-IIc (Slc34a3), are located in the brush border membrane (BBM) of the proximal tubule and mediate renal phosphate reabsorption. Transcriptome analysis of kidneys from acid-loaded mice revealed a large decrease in NaPi-IIc messenger RNA (mRNA) and a smaller reduction in NaPi-IIa mRNA abundance. To investigate the contribution of transporter regulation to phosphaturia during MA, we examined renal phosphate transporters in normal and Slc34a1-gene ablated (NaPi-IIa KO) mice acid-loaded for 2 and 7 days. In normal mice, urinary phosphate excretion was transiently increased after 2 days of acid loading, whereas no change was found in Slc34a1−/− mice. BBM Na/Pi cotransport activity was progressively and significantly decreased in acid-loaded KO mice, whereas in WT animals, a small increase after 2 days of treatment was seen. Acidosis increased BBM NaPi-IIa abundance in WT mice and NaPi-IIc abundance in WT and KO animals. mRNA abundance of NaPi-IIa and NaPi-IIc decreased during MA. Immunohistochemistry did not indicate any change in the localization of NaPi-IIa and NaPi-IIc along the nephron. Interestingly, mRNA abundance of both Slc20 phosphate transporters, Pit1 and Pit2, was elevated after 7 days of MA in normal and KO mice. These data demonstrate that phosphaturia during acidosis is not caused by reduced protein expression of the major Na/Pi cotransporters NaPi-IIa and NaPi-IIc and suggest a direct inhibitory effect of low pH mainly on NaPi-IIa. Our data also suggest that Pit1 and Pit2 transporters may play a compensatory role.






Similar content being viewed by others
References
Ambuhl PM, Zajicek HK, Wang H, Puttaparthi K, Levi M (1998) Regulation of renal phosphate transport by acute and chronic metabolic acidosis in the rat. Kidney Int 53:1288–1298
Amstutz M, Mohrmann M, Gmaj P, Murer H (1985) Effect of pH on phosphate transport in rat renal brush border membrane vesicles. Am J Physiol 248:F705–F710
Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U SA 95:5372–5377
Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H (2006) SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78:179–192
Berthelot M (1859) Violet d’aniline. Rep Chim App 1:284
Biber J, Stieger B, Haase W, Murer H (1981) A high yield preparation for rat kidney brush border membranes. Different behaviour of lysosomal markers. Biochim Biophys Acta 647:169–176
Biber J, Stieger B, Stange G, Murer H (2007) Isolation of renal proximal tubular brush-border membranes. Nature protocols 2:1356–1359
Brown AJ, Finch J, Slatopolsky E (2002) Differential effects of 19-nor-1,25-dihydroxyvitamin D(2) and 1,25-dihydroxyvitamin D(3) on intestinal calcium and phosphate transport. J Lab Clin Med 139:279–284
Busch AE, Wagner CA, Schuster A, Waldegger S, Biber J, Murer H, Lang F (1995) Properties of electrogenic Pi transport by a human renal brush border Na+/Pi transporter. J Am Soc Nephrol 6:1547–1551
Custer M, Lotscher M, Biber J, Murer H, Kaissling B (1994) Expression of Na-P(i) cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol 266:F767–F774
Dawson TP, Gandhi R, Le Hir M, Kaissling B (1989) Ecto-5′-nucleotidase: localization in rat kidney by light microscopic histochemical and immunohistochemical methods. J Histochem Cytochem 37:39–47
Guntupalli J, Eby B, Lau K (1982) Mechanism for the phosphaturia of NH4Cl: dependence on acidemia but not on diet PO4 or PTH. Am J Physiol 242:F552–F560
Gupta A, Tenenhouse HS, Hoag HM, Wang D, Khadeer MA, Namba N, Feng X, Hruska KA (2001) Identification of the type II Na(+)-Pi cotransporter (Npt2) in the osteoclast and the skeletal phenotype of Npt2−/− mice. Bone 29:467–476
Hattenhauer O, Traebert M, Murer H, Biber J (1999) Regulation of small intestinal Na–P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol 277:G756–G762
Kavanaugh MP, Kabat D (1996) Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int 49:959–963
Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, Miller AD (1994) Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A 91:7071–7075
Lemann J Jr., Bushinsky DA, Hamm LL (2003) Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 285:F811–F832
Levi M, Lotscher M, Sorribas V, Custer M, Arar M, Kaissling B, Murer H, Biber J (1994) Cellular mechanisms of acute and chronic adaptation of rat renal P(i) transporter to alterations in dietary P(i). Am J Physiol 267:F900–F908
Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM (2006) Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78:193–201
Madjdpour C, Bacic D, Kaissling B, Murer H, Biber J (2004) Segment specific expression of sodium-phosphate cotransporters NaPi-IIa and -IIc and interacting proteins in mouse renal proximal tubules. Pflugers Arch 448:402–410
Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H (2005) Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial 9:331–335
Murer H, Forster I, Biber J (2004) The sodium phosphate cotransporter family SLC34. Pflugers Arch 447:763–767
Murer H, Hernando N, Forster I, Biber J (2003) Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol 65:531–542
Murer H, Hernando N, Forster I, Biber J (2000) Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80:1373–1409
Nowik M, Lecca MR, Velic A, Rehrauer H, Brandli AW, Wagner CA (2008) Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32:322–334
Ohkido I, Segawa H, Yanagida R, Nakamura M, Miyamoto K (2003) Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch 446:106–115
Olah Z, Lehel C, Anderson WB, Eiden MV, Wilson CA (1994) The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem 269:25426–25431
Radanovic T, Wagner CA, Murer H, Biber J (2005) Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288:G496–G500
Seaton B, Ali A (1984) Simplified manual high performance clinical chemistry methods for developing countries. Med Lab Sci 41:327–336
Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K (2002) Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277:19665–19672
Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, Saito H, Fukushima N, Miyamoto K (2003) Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflugers Arch 446:585–592
Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K (2005) Internalization of renal type IIc Na–Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol 288:F587–F596
Stauber A, Radanovic T, Stange G, Murer H, Wagner CA, Biber J (2005) Regulation of intestinal phosphate transport. II. Metabolic acidosis stimulates Na(+)-dependent phosphate absorption and expression of the Na(+)-P(i) cotransporter NaPi-IIb in small intestine. Am J Physiol Gastrointest Liver Physiol 288:G501–G506
Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K (2003) Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol 285:F1271–F1278
Tenenhouse HS, Roy S, Martel J, Gauthier C (1998) Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am J Physiol 275:F527–F534
Virkki LV, Forster IC, Biber J, Murer H (2005) Substrate interactions in the human type IIa sodium-phosphate cotransporter (NaPi-IIa). Am J Physiol Renal Physiol 288:F969–F981
Zajicek HK, Wang H, Puttaparthi K, Halaihel N, Markovich D, Shayman J, Beliveau R, Wilson P, Rogers T, Levi M (2001) Glycosphingolipids modulate renal phosphate transport in potassium deficiency. Kidney Int 60:694–704
Acknowledgments
M. Nowik is the recipient of a PhD student fellowship from the Zurich Center for Integrative Human Physiology. This study was supported by grants from the Swiss National Science Research Foundation to J. Biber and H. Murer (31-65397.01) and C. A. Wagner (31-109677/1) and the 6th EU Frame work project EuReGene to J. Biber, H. Murer, and C. A. Wagner. The use of the ZIHP Core Facility for Rodent Physiology is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Characterization of the anti-NaPi-IIc antibody by immunoblotting. Brush border membrane vesicles were prepared from kidneys of Slc34a1+/− and Slc34a1−/− mice to test the specificity and cross-reactivity of new antibodies against NaPi-IIc. The antibody against mouse NaPi-IIc was raised in rabbit, affinity-purified, and recognized a single major band of approximately 75kDa (stripe B). This band was prevented by preincubation with the immunizing peptide (stripe C). The preimmune serum from the rabbit used to obtain the anti-NaPi-IIc antibody did not show any reactivity (stripe D). Stripe A shows NaPi-IIa staining in the same samples (PDF 50 KB).
Supplementary Fig. 2
Characterization of anti-NaPi-IIc antibody by immunohistochemistry. Serial kidney sections of normal mice were stained with antibodies against NaPi-IIa (left panel), NaPi-IIc (middle panel), and NaPi-IIc after preincubation with the immunizing peptide (right panel). No staining was detected with the anti-NaPi-IIc antibody after preincubation with the immunizing peptide. Please note: The background level was enhanced in the right panel to detect possible unspecific staining (PDF 342 KB).
Supplementary Fig. 3
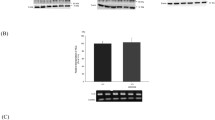
Increased abundance of NaPi-IIc protein in the brush border membrane of kidneys from NaPi-IIa-deficient mice. Blots were probed for NaPi-IIc and β-actin, and data are summarized by normalizing NaPi-IIc abundance against β-actin (mean ± SEM). These data are in agreement with previous work demonstrating that renal NaPi-IIc protein expression is significantly increased in NaPi-Iia-deficient mice when compared with normal littermates [34] (PDF 29 KB).
Rights and permissions
About this article
Cite this article
Nowik, M., Picard, N., Stange, G. et al. Renal phosphaturia during metabolic acidosis revisited: molecular mechanisms for decreased renal phosphate reabsorption. Pflugers Arch - Eur J Physiol 457, 539–549 (2008). https://doi.org/10.1007/s00424-008-0530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-008-0530-5