Abstract
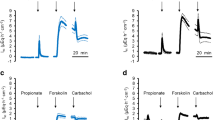
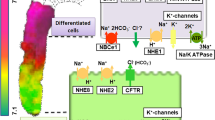
Short-chain fatty acids (SCFAs) produced by the microbial fermentation of undigested polysaccharide are rapidly absorbed in the large intestine. One proposed mechanism for this SCFA absorption is \( {{\text{SCFA}}} \mathord{\left/ {\vphantom {{{\text{SCFA}}} {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} }}} \right. \kern-\nulldelimiterspace} {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \) exchange. To provide factual evidence for the operation of \( {{\text{SCFA}}} \mathord{\left/ {\vphantom {{{\text{SCFA}}} {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} }}} \right. \kern-\nulldelimiterspace} {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \) exchange, we mounted an isolated mouse cecum in the Ussing chamber and measured the rates of propionate absorption (Jprop(ms)), alkaline secretion (JOH(sm)) and total \( {\text{CO}}_{{\text{2}}} {\left( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} {\text{ + CO}}_{{\text{2}}} } \right)} \) secretion (JtCO2(sm)), and the short-circuit current (Isc) with the mucosal side bathed with a Cl− and \( {\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \)-free solution. In the presence of propionate only on the mucosal but not in the serosal solution, Jprop(ms) was larger when the serosal side was bathed with a \( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \mathord{\left/ {\vphantom {{{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } {{\text{CO}}_{{\text{2}}} }}} \right. \kern-\nulldelimiterspace} {{\text{CO}}_{{\text{2}}} } \)-containing solution than with a \( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \mathord{\left/ {\vphantom {{{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } {{\text{CO}}_{{\text{2}}} }}} \right. \kern-\nulldelimiterspace} {{\text{CO}}_{{\text{2}}} } \)-free solution. The addition of propionate to the mucosal side caused an increase in JOH(sm) and JtCO2(sm), the magnitude of these increases both being much greater with the serosal side bathed with the \( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \mathord{\left/ {\vphantom {{{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } {{\text{CO}}_{{\text{2}}} }}} \right. \kern-\nulldelimiterspace} {{\text{CO}}_{{\text{2}}} } \)-containing solution than with the \( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \mathord{\left/ {\vphantom {{{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } {{\text{CO}}_{{\text{2}}} }}} \right. \kern-\nulldelimiterspace} {{\text{CO}}_{{\text{2}}} } \)-free solution. Acetazolamide, a carbonic anhydrase inhibitor, largely suppressed \( {\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \)-dependent components of Jprop(ms), propionate-induced JOH(sm), and propionate-induced JtCO2(sm). Acetazolamide, however, did not affect Isc. The \( {\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \)-dependent component of Jprop(ms) was not inhibited by either lactate or α-cyano-4-hydroxycinnamate, a typical substrate and an inhibitor of the monocarboxylate transporter (MCT1), respectively. It is concluded that an electroneutral, carbonic anhydrase-dependent \( {{\text{SCFA}}} \mathord{\left/ {\vphantom {{{\text{SCFA}}} {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} }}} \right. \kern-\nulldelimiterspace} {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \) exchange mechanism was involved in SCFA absorption. The apical membrane protein for this pathway is not MCT1 and remains to be determined.








Similar content being viewed by others
Notes
Isc occurring under the present experimental conditions could have mainly been due to diffusion via the paracellular pathway of Cl−, \( {\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \), propionate− and/or HEPES−, whose concentrations were different between the mucosal and serosal solutions. However, the contributions of \( {\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \) and propionate− to Isc would have been minimal since JOH(sm) and Jprop(ms) were not affected by changing the transmural potential difference (see “Effect on JOH(sm) and Jprop(ms) of changing the transmural potential difference” in the Results section). An Isc component due to active ion transport would also be minimal, since, when both the mucosal and serosal sides were bathed with the \( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \mathord{\left/ {\vphantom {{{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } {{\text{CO}}_{{\text{2}}} }}} \right. \kern-\nulldelimiterspace} {{\text{CO}}_{{\text{2}}} } \)-buffered solutions, Isc was less than 10 μA·cm−2 in the presence of TTX and indomethacin in the mouse cecum (Kawamata, K., unpublished observation).
References
Allen A, Flemstrom G (2005) Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288:C1–C19
Bugaut M (1987) Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86:439–472
Campbell JM, Fahey GC Jr, Wolf BW (1997) Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127:130–136
Charney AN, Micic L, Egnor RW (1998) Nonionic diffusion of short-chain fatty acids across rat colon. Am J Physiol 274:G518–G524
Chu S, Montrose MH (1996) Non-ionic diffusion and carrier-mediated transport drive extracellullar pH regulation of mouse colonic crypts. J Physiol 494(Pt 3):783–793
Dohgen M, Hayahshi H, Yajima T, Suzuki Y (1994) Stimulation of bicarbonate secretion by luminal short-chain fatty acid in the rat and human colon in vitro. Jpn J Physiol 44:519–531
Fleming SE, Fitch MD, Chansler MW (1989) High-fiber diets: influence on characteristics of cecal digesta including short-chain fatty acid concentrations and pH. Am J Clin Nutr 50:93–99
Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS (1994) Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76:865–873
Genz AK, von Engelhardt W, Busche R (1999) Maintenance and regulation of the pH microclimate at the luminal surface of the distal colon of guinea-pig. J Physiol 517(Pt 2):507–519
Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K (2000) Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279:G775–G780
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628
Halperin ML (1982) Metabolism and acid–base physiology. Artif Organs 6:357–362
Harig JM, Ng EK, Dudeja PK, Brasitus TA, Ramaswamy K (1996) Transport of n-butyrate into human colonic luminal membrane vesicles. Am J Physiol 271:G415–G422
Hochachka PW, Mommsen TP (1983) Protons and anaerobiosis. Science 219:1391–1397
Kivela A, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, Tureci O, Rajaniemi H (2000) Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol 156:577–584
Kivela AJ, Kivela J, Saarnio J, Parkkila S (2005) Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol 11:155–163
Leng E (1978) Absorption of inorganic ions and volatile fatty acids in the rabbit caecum. Br J Nutr 40:509–519
Macfarlane GT, Cummings JH (1991) The colonic flora, fermentation, and large bowel digestive function. In: Phillips SF, Pemberton JH, Shorter RG (eds) The large intestine. Raven Press, New York, pp 51–92
Mascolo N, Rajendran VM, Binder HJ (1991) Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology 101:331–338
Montrose MH, Chu S (1997) Transepithelial SCFA gradients regulate polarized Na/H exchangers and pH microdomains in colonic epithelia. Comp Biochem Physiol A Physiol 118:389–393
Ritzhaupt A, Ellis A, Hosie KB, Shirazi-Beechey SP (1998) The characterization of butyrate transport across pig and human colonic luminal membrane. J Physiol 507(Pt 3):819–830
Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP (1998) Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport l–lactate as well as butyrate. J Physiol 513(Pt 3):719–732
Schultz S (1980) Basic principles of membrane transport. (IUPAB biophysical series; 2) Cambridge University Press, New York, pp 28–29
Sellin JH, DeSoignie R (1990) Short-chain fatty acid absorption in rabbit colon in vitro. Gastroenterology 99:676–683
Sellin JH, DeSoignie R, Burlingame S (1993) Segmental differences in short-chain fatty acid transport in rabbit colon: effect of pH and Na. J Membr Biol 136:147–158
Sellin JH, De Soignie R (1998) Short-chain fatty acids have polarized effects on sodium transport and intracellular pH in rabbit proximal colon. Gastroenterology 114:737–747
Sellin JH (1999) SCFAs: the enigma of weak electrolyte transport in the colon. News Physiol Sci 14:58–64
Stein J, Schroder O, Milovic V, Caspary WF (1995) Mercaptopropionate inhibits butyrate uptake in isolated apical membrane vesicles of the rat distal colon. Gastroenterology 108:673–679
Stein J, Zores M, Schroder O (2000) Short-chain fatty acid (SCFA) uptake into Caco-2 cells by a pH-dependent and carrier mediated transport mechanism. Eur J Nutr 39:121–125
Stevens CE, Hume ID (1998) Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev 78:393–427
Umesaki Y, Yajima T, Yokokura T, Mutai M (1979) Effect of organic acid absorption on bicarbonate transport in rat colon. Pflugers Arch 379:43–47
Vidyasagar S, Rajendran VM, Binder HJ (2004) Three distinct mechanisms of HCO3- secretion in rat distal colon. Am J Physiol Cell Physiol 287:C612–C621
Vidyasagar S, Barmeyer C, Geibel J, Binder HJ, Rajendran VM (2005) Role of short-chain fatty acids in colonic HCO(3) secretion. Am J Physiol Gastrointest Liver Physiol 288:G1217–G1226
von Engelhardt W, Burmester M, Hansen K, Becker G, Rechkemmer G (1993) Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. J Physiol 460:455–466
von Engelhardt W, Gros G, Burmester M, Hansen K, Becker G, Rechkemmer G (1994) Functional role of bicarbonate in propionate transport across guinea-pig isolated caecum and proximal colon. J Physiol 477(Pt 2):365–371
von Engelhardt W, Busche R, Burmester M, Hansen K, Becker G (1997) Transport of propionate across the distal colonic epithelium of guinea pig in the presence and absence of bicarbonate and of chloride. Zentralbl Veterinarmed A 44:73–78
Acknowledgment
We thank Tony Innes of Link Associates for helping us to edit the English text.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
Effect of inhibitors on propionate-induced JOH(sm) in the presence of serosal \( {{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } \mathord{\left/ {\vphantom {{{\text{HCO}}^{{\text{ - }}}_{{\text{3}}} } {{\text{CO}}_{{\text{2}}} }}} \right. \kern-\nulldelimiterspace} {{\text{CO}}_{{\text{2}}} } \) (PDF 54 kb)
Supplementary Figure
Effect of changing the transmural potential difference. The mucosal side was bathed with the buffer-free solution containing 25 mM propionate, and the serosal side was bathed with the \( {\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \)-buffered solution. The transmural potential difference was clamped at 0 mV and ±20 mV by using the same set-up as that for the short-circuit current measurements. JOH(sm) was determined by a pH stat and Jprop(ms) was determined by HPLC. The JOH(sm) and Jprop(ms) values at a 0-mV transmural potential difference were determined for 90 min, before the potential difference was clamped at +20 mV (the mucosal potential as referred to the serosal side) (a), or at −20 mV (b) and JOH(sm) and Jprop(ms) were again determined during the following 60 min. The experiments at +20 mV and −20 mV were each done on two adjacent tissues obtained from the same animal and repeated on five animals (PDF 35 kb)
Rights and permissions
About this article
Cite this article
Kawamata, K., Hayashi, H. & Suzuki, Y. Propionate absorption associated with bicarbonate secretion in vitro in the mouse cecum. Pflugers Arch - Eur J Physiol 454, 253–262 (2007). https://doi.org/10.1007/s00424-006-0200-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0200-4