Abstract
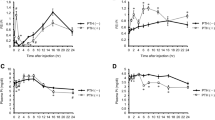
Coexpression studies in Xenopus oocytes revealed the ability of the serum- and glucocorticoid-inducible kinase 1 (SGK1) to stimulate the renal epithelial Ca2+ channel TRPV5. SGK1 increases the abundance of the channel protein in the plasma membrane, an effect requiring the participation of the Na+/H+ exchanger regulating factor 2 (NHERF2). The present study was performed to explore the role of SGK1 in the regulation of renal Ca2+ handling in vivo. To this end, TRPV5, calbindin D-28K abundance, and renal Ca2+ excretion were analyzed in gene-targeted mice lacking functional SGK1 (sgk1 −/−) and their age- and sex-matched littermates (sgk1 +/+). Immunohistochemistry revealed lower abundance of TRPV5 and calbindin D-28K protein in sgk1 −/− mice than in sgk1 +/+ mice, both fed with control diet. Feeding the mice a Ca2+-deficient diet marked ly increased TRPV5 protein abundance in both genotypes. Renal Ca2+ excretion under control diet was significantly lower in sgk1 −/− than in sgk1 +/+ mice. The Ca2+-deficient diet decreased renal excretion of Ca2+ to the same levels in both phenotypes. Furosemide increased fractional Ca2+ excretion and dissipated the difference between phenotypes. We conclude that lack of SGK1 may lead to decrease in TRPV5 abundance in connecting tubules but does not abrogate TRPV5 regulation. The decrease in abundance of TRPV5 in connecting tubules of sgk1 −/− mice is presumably compensated for by enhanced Ca2+ reabsorption in upstream nephron segments such as the loop of Henle, which may indirectly result from impaired SGK1-dependent Na+ reabsorption in the aldosterone-sensitive distal part of the nephron, salt loss, and enhanced Na+ (and Ca2+) reabsorption in those upstream nephron segments.





Similar content being viewed by others
References
Akutsu N, Lin R, Bastien Y, Bestawros A, Enepekides DJ, Black MJ, White JH (2001) Regulation of gene expression by 1alpha,25-dihydroxyvitamin D3 and its analog EB1089 under growth-inhibitory conditions in squamous carcinoma cells. Mol Endocrinol 15:1127–1139
Alessi DR, Cohen P (1998) Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8:55–62
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551
Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D (1999) Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A 96:2514–2519
Divecha N, Banfic H, Irvine RF (1991) The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J 10:3207–3214
Embark HM, Setiawan I, Poppendieck S, van de Graaf SFJ, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun CC, Bindels RJM, Lang F (2004) Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem 14:203–212
Firestone GL, Giampaolo JR, O’Keeffe BA (2003) Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem 13:1–12
Friedman PA, Gesek FA (1995) Cellular calcium transport in renal epithelia: measurement, mechanisms, and regulation. Physiol Rev 75:429–471
Gamper N, Fillon S, Huber SM, Feng Y, Kobayashi T, Cohen P, Lang F (2002) IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1. Pflugers Arch 443:625–634
Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, van Os CH, Arnaud R, Bindels RJ (2002) Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J 16:1398–1406
Hoenderop JG, Muller D, Suzuki M, van Os CH, Bindels RJ (2000) Epithelial calcium channel: gate-keeper of active calcium reabsorption. Curr Opin Nephrol Hypertens 9:335–340
Hoenderop JG, Nilius B, Bindels RJ (2002) Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol 64:529–549
Hoenderop JG, Van Der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ (1999) Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274:8375–8378
Hoenderop JG, Van Der Kemp AW, Hartog A, van Os CH, Willems PH, Bindels RJ (1999) The epithelial calcium channel, ECaC, is activated by hyperpolarization and regulated by cytosolic calcium. Biochem Biophys Res Commun 261:488–492
Hoenderop JG, Willems PH, Bindels RJ (2000) Toward a comprehensive molecular model of active calcium reabsorption. Am J Physiol Renal Physiol 278:F352–F360
Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V (2004) Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15:885–891
Kobayashi T, Cohen P (1999) Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339:319–328
Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F et al (1994) Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J 13:2313–2321
Mery L, Strauss B, Dufour JF, Krause KH, Hoth M (2002) The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 115:3497–3508
Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G (1999) Sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem 274:16973–16978
Palmada M, Poppendieck S, Embark HM, van de Graaf SF, Boehmer C, Bindels RJ, Lang F (2005) Requirement of PDZ domains for the stimulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase SGK1. Cell Physiol Biochem 15:175–182
Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA (1999) Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18:3024–3033
Pearce D (2003) SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem 13:13–20
Shenolikar S, Weinman EJ (2001) NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol 280:F389–F395
Shigaev A, Asher C, Latter H, Garty H, Reuveny E (2000) Regulation of sgk by aldosterone and its effects on the epithelial Na(+) channel. Am J Physiol Renal Physiol 278:F613–F619
Vallon V (2003) In vivo studies of the genetically modified mouse kidney. Nephron Physiol 94:1–5
Verrey F, Loffing J, Zecevic M, Heitzmann D, Staub O (2003) SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell Physiol Biochem 13:21–28
Webster MK, Goya L, Firestone GL (1993) Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J Biol Chem 268:11482–11485
Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL (1993) Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13:2031–2040
Weinman EJ, Steplock D, Wang Y, Shenolikar S (1995) Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)–H+ exchanger. J Clin Invest 95:2143–2149
Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D (2002) Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest 110:1263–1268
Acknowledgements
This work was supported by grants from DFG and BMBF (F.L. and V.V.) and the DAAD (O.N.).
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Sandulace and F. Grahammer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sandulache, D., Grahammer, F., Artunc, F. et al. Renal Ca2+ handling in sgk1 knockout mice. Pflugers Arch - Eur J Physiol 452, 444–452 (2006). https://doi.org/10.1007/s00424-005-0021-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-005-0021-x