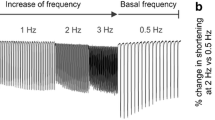
Abstract
β-Adrenoceptor/cAMP-dependent Ser16-phosphoryation as well as Ca2+-dependent Thr17-phosphorylation of phospholamban (PLB) influences SERCA 2a activity and thus myocardial contractility. To determine the cross-signaling between Ca2+ and cAMP pathways, the phosphorylation of Ser16-PLB and Thr17-PLB was studied at increasing stimulation frequencies as well as in the presence of β-adrenergic stimulation in isolated ventricular trabeculae from failing (dilative cardiomyopathy, DCM, heart transplants, n=9) and non-failing human myocardium (donor hearts, NF, n=9). In addition, we measured the intracellular Ca2+-transient (fura-2) at increasing stimulation frequencies (0.5–3.0 Hz). Protein expression of SERCA 2a and phospholamban was similar in DCM and NF. In DCM, diastolic [Ca2+]i was increased and systolic [Ca2+]i as well as Ser16 PLB-phosphorylation were decreased as compared to NF at 0.5 Hz. The positive force–frequency relationship in human non-failing myocardium was accompanied by a frequency-dependent increase in Ser16-PLB, but not Thr17-PLB phosphorylation. In DCM, Ser16-PLB as well as Thr17-PLB phosphorylation were not altered at higher stimulation frequencies. After application of isoprenaline (1 µM), a profound increase in Ser16-PLB phosphorylation was accompanied by a small increase in Thr17-PLB phosphorylation, only in NF. The frequency-dependent phosphorylation of Ser16-PLB may favor an increase in Ca2+ transient and force generation in humans. Cross talk signaling of Ser16/Thr17-PLB phosphorylation after β-adrenergic stimulation exists in non-failing, but not in failing human myocardium. The Ca2+-dependent CaM-kinase activity may be altered in human heart failure.




Similar content being viewed by others
References
Bartel S, Vetter D, Schlegel WP, Wallukat G, Krause EG, Karczewski P (2000) Phosphorylation of phospholamban at threonine-17 in the absence and presence of beta-adrenergic stimulation in neonatal rat cardiomyocytes. J Mol Cell Cardiol 32:2173–2185
Beuckelmann DJ, Nabauer M, Erdmann E (1992) Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85:1046–1055
Bluhm WF, Kranias EG, Dillmann WH, Meyer M (2000) Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol 278:H249–H255
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brixius K, Pietsch M, Hoischen S, Muller-Ehmsen J, Schwinger RHG (1997) Effect of inotropic interventions on contraction and Ca2+ transients in the human heart. J Appl Physiol 83:652–660
DeSantiago J, Maier LS, Bers DM (2002) Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol 34:975–984
Drago GA, Colyer J (1994) Discrimination between two sites of phosphorylation on adjacent amino acids by phosphorylation site-specific antibodies to phospholamban. J Biol Chem 269:25073–25077
Feldman MD, Phillips P, Schoen F, Morgan JP (1988) Reversal of the force-frequency-relationship in working myocardium from patients with endstage heart failure. J Appl Cardiol 3:273–283
Frank KF, Tilgmann C, Shannon TR, Bers DM, Kranias EG (2000) Regulatory role of phospholamban in the efficiency of cardiac sarcoplasmic reticulum Ca2+ transport. Biochemistry 39:14176–14182
Fujii J, Maruyama K, Tada M, MacLennan DH (1990) Co-expression of slow-twitch/cardiac muscle Ca2+-ATPase (SERCA2) and phospholamban. FEBS Lett 273:232–234
Fujii J, Ueno A, Kitano K, Tanaka S, Kadoma M, Tada M (1987) Complete complementary DNA-derived amino acid sequence of canine cardiac phospholamban. J Clin Invest 79:301–304
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao R-P (2000) Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem 275:22532–22536
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H (1994) Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75:434–442
Jackson WA, Colyer J (1996) Translation of Ser16 and Thr17 phosphorylation of phospholamban into Ca2+-pump stimulation. Biochem J 316:201–207
Kranias EG, Gupta RC, Jakab G, Kim HW, Steenaart NA, Rapundalo ST (1988) The role of protein kinases and protein phosphatases in the regulation of cardiac sarcoplasmic reticulum function. Mol Cell Biochem 82:37–44
Luo W, Chu G, Sato Y, Zhou Z, Kadambi VJ, Kranias EG (1998) Transgenic approaches to define the functional role of dual site phospholamban phosphorylation. J Biol Chem 273:4734–4739
Mattiazi A, Vittone L, Mundina-Weilenmann C, Said M (1998) Phosphorylation of the Thr17 residue of phospholamban. New insights into the physiological role of the CaMK-II pathway of phospholamban phosphorylation. Ann N Y Acad Sci 853:280–283
Meyer M, Bluhm W, He H, Post SR, Giordano FJ, Lew WY, Dillman WH (1999) Phospholamban-to-SERCA2 ratio controls the force-frequency relationship. Am J Physiol 276:H779–H785
Munch G, Bolck B, Brixius K, Reuter H, Mehlhorn U, Bloch W, Schwinger RHG (2000) SERCA2a activity correlates with the force-frequency relationship in human myocardium. Am J Physiol 278:H1924–H1932
Munch G, Bolck B, Hoischen S, Brixius K, Bloch W, Reuter H, Schwinger RHG (1998) Unchanged protein expression of sarcoplasmic reticulum Ca2+-ATPase, phospholamban, and calsequestrin in terminally failing human myocardium. J Mol Med 76:434–441
Mundina-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A (1996) Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem 271:33561–33567
Netticadan T, Temsah RM, Kawabata K, Dhalla NS (2002) Ca2+-overload inhibits the cardiac SR Ca2+-calmodulin protein kinase activity. Biochem Biophys Res Commun 293:727–732
Reddy LG, Jones LR, Pace RC, Stokes DL (1996) Purified, reconstituted cardiac Ca2+-ATPase is regulated by phospholamban but not by direct phosphorylation with Ca2+/calmodulin- dependent protein kinase. J Biol Chem 271:14964–14970
Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK (1998) Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol 30:1929–1937
Schwinger RHG, Bohm M, Erdmann E (1992) Inotropic and lusitropic dysfunction in myocardium from patients with dilated cardiomyopathy. Am Heart J 123:116–128
Schwinger RHG, Bohm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, Krause EG, Erdmann E (1995) Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 92:3220–3228
Schwinger RHG, Brixius K, Bavendiek U, Hoischen S, Muller-Ehmsen J, Bolck B, Erdmann E (1997) Effect of cyclopiazonic acid on the force-frequency relationship in human nonfailing myocardium. J Pharmacol Exp Ther 283:286–292
Schwinger RHG, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E (1999) Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol 31:479–491
Simmerman HK, Collins JH, Theibert JL., Wegener AD, Jones LR (1986) Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem 261:13333–13341
Swank RT, Munkres KD (1971) Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem 39:462–477
Towbin H, Stähelein T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vittone L, Mundina-Weilenmann C, Said M, Ferrero P, Matiazzi A (2002) Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol 34:39–50
Vittone L, Mundina-Weilenmann C, Said M, Mattiazzi A (1998) Mechanisms involved in the acidosis enhancement of the isoproterenol- induced phosphorylation of phospholamban in the intact heart. J Biol Chem 273:9804–98011
Wegener AD, Simmerman HK, Lindemann JP, Jones LR (1989) Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem 264:11468–11474
Acknowledgements
We are indebted to all colleges of the Department of the Cardiothoracic Surgery of the University of Cologne (Director: Professor Dr. R. E. de Vivie) for providing us with human myocardial samples. The authors thank Mrs. Katja Rössler, Esra Köroglu and Kerstin Schenk for their excellent technical help. This study was supported by the Deutsche Forschungsgemeinschaft and the Köln Fortune Program/faculty of Medicine, University of Cologne. This work contains parts of the doctoral thesis of A.W.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brixius, K., Wollmer, A., Bölck, B. et al. Ser16-, but not Thr17-phosphorylation of phospholamban influences frequency-dependent force generation in human myocardium. Pflugers Arch - Eur J Physiol 447, 150–157 (2003). https://doi.org/10.1007/s00424-003-1163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-003-1163-3