Abstract
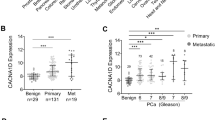
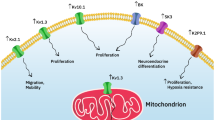
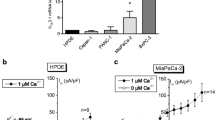
Voltage-gated K+ currents expressed in two rat prostate cancer ('Dunning') cell lines of markedly different metastatic ability were characterised using electrophysiological, pharmacological and molecular approaches. Whole-cell patch-clamp recordings showed that both strongly metastatic MAT-LyLu and weakly metastatic AT-2 cell lines possessed outward (delayed-rectifier type) K+ currents, which activated at around −40 mV. From the parameters measured, several characteristics of the two cell lines were similar. However, a number of statistically significant differences were noted for MAT-LyLu versus the AT-2 cells as follows: (1) current densities were smaller; (2) the slope factor for channel activation was smaller; (3) the voltage at which current was half-inactivated, and the slope factor for channel inactivation were greater; (4) the time constants for current decay at −20 and 0 mV were smaller; and (5) the residual peak current was larger following 60 s of repetitive voltage pulses for stimulation frequencies in the range 0.05–0.2 Hz. On the other hand, the K+ currents in both cell lines showed similar pharmacological profiles. Thus, the currents were blocked by 4-aminopyridine, tetraethylammonium, verapamil, margatoxin, and charybdotoxin, with highly similar IC50s for given blockers. The electrophysiological and pharmacological data taken together suggested expression of voltage-gated K+ channels of the Kv1 family, expression of the Kv1.3 subunit being predominant. Western blot and RT-PCR tests both confirmed that the cells indeed expressed Kv1.3 and to a lesser extent Kv1.4 and Kv1.6 channel α-subunits. In view of the similarity of channel expression in the two cell lines, voltage-gated K+ channel activity may not be a primary determinant of metastatic potential in the rat model of prostate cancer, but the possible contribution of K+ channel activity to the metastatic process is discussed.






Similar content being viewed by others
References
BekeleArcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS (1996) Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology 35:851–865
Blandino JKW, Viglione MP, Bradley WA, Oie HK, Kim YI (1995) Voltage-dependent sodium channels in human small-cell lung cancer cells: role in action potentials and inhibition by Lambert-Eaton syndrome IgG. J Membr Biol 143:153–163
Caffrey JM, Brown AM, Schneider MD (1987) Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science 236:570–573
Chen C, Corbley MJ, Roberts TM, Hess P (1988) Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science 239:1024–1026
Chin LS, Park CC, Zitnay KM, Sinha M, DiPatri AJ, Perillán P, Simard JM (1997) 4-aminopyridine causes apoptosis and blocks an outward rectifier K+ channel in malignant astrocytoma cell lines. J Neurosci Res 48:122–127
Chiu SY, Wilson GF (1989) The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J Physiol (Lond) 408:199–222
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
DeCoursey TE, Chandy KG, Gupta S, Cahalan MD (1984) Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis. Nature 307:465–468
Diss JKJ, Archer SN, Hirano J, Fraser SP, Djamgoz MBA (2001) Expression profiles of voltage-gated Na+ channel α-subunit genes rat and human prostate cancer cell lines. Prostate 48:1–14
Douglass J, Osborne PB, Cai YC, Wilkinson M, Christie NJ, Adelman JP (1990) Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel. J Immunol 144:4841–4850
Fitzsimmons SA, Workman P, Grever M, Paull K, Camalier R, Lewis AD (1996) Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst 88:259–269
Fraser SP, Salvador V, Djamgoz MBA (1998) Voltage-gated Na+ channel activity contributes to rodent prostate cancer migration in vitro. J Physiol (Lond) 513:131P
Fraser SP, Ding Y, Liu A, Foster CS, Djamgoz MBA (1999) Tetrodotoxin suppresses morphological enhancement of the metastatic MAT-LyLu rat prostate cancer cell line. Cell Tissue Res 295:505–512
Fraser SP, Grimes JA, Djamgoz MBA (2000) Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate 44:61–76
Frech GC, VanDongen AMJ, Schuster G, Brown AM, Joho RH (1989) A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature 340:642–645
Garcia ML, Galvez A, Garcia-Calvo M, King VF, Vazquez J, Kaczorowski GJ (1991) Use of toxins to study potassium channels. J Bioenerg Biomembr 23:615–646
Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Scmalhofer W, Kaczorowski GJ, Garcia ML (1993) Purification, characterization, and biosynthesis of margatoxin, a component of Centruoides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem 268:18866–18874
Grimes JA, Djamgoz MBA (1998) Electrophysiological characterization of voltage-gated Na+ current expressed in the highly metastatic Mat-LyLu cell line of rat prostate cancer. J Cell Physiol 175:50–58
Grimes JA, Fraser SP, Stephens GJ, Downing JEG, Laniado ME, Foster CS, Abel PD, Djamgoz MBA (1995) Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS Lett 369:290–294
Hamann M, Widmer H, Baroffio A, Aubry JP, Krause RM, Kaelin A, Bader CR (1994) Sodium and potassium currents in freshly isolated and in proliferating human muscle satellite cells. J Physiol (Lond) 475:305–317
Hille B (1992) Ionic channels of excitable membranes, 2nd edn. Sinauer, Sunderland, Mass
Isaacs JT, Isaacs WT, Feitz WFJ, Scheres J (1986) Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate 9:261–281
Isacoff EY, Jan YN, Jan LY (1990) Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature 345:530–534
Itoh K, Stevens B, Schachner R, Fields RD (1995) Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science 27:1369–1372
Kupper J, Bowlby MR, Marom S, Levitan IB (1995) Intracellular and extracellular amino acids that influence C-type inactivation and its modulation in a voltage-dependent potassium channel. Pflugers Arch 430:1–11
Lang F, Rehwald W (1992) Potassium channels in renal epithelial transport regulation. Physiol Rev 72:1–32
Laniado ME, Lalani E-N, Fraser SP, Grimes JA, Bhangal G, Djamgoz MBA, Abel PD (1997) Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasiveness in vitro. Am J Pathol 150:1213–1221
Laniado ME, Fraser SP, Djamgoz MBA (2001) Voltage-gated K+ channel activity in human prostate cancer cell lines of markedly different metastatic potential: Distinguishing characteristics of PC-3 and LNCaP cells. Prostate 45:1–15
Lee SC, Price M, Prystowsky MB, Deutsch C (1988) Volume response of quiescent and interleukin 2-stimulated T-lymphocytes to hypotonicity. Am J Physiol 254:C286–296
Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O (2000) Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta 1 integrins. J Exp Med 191:1167–1176
MacKinnon R, Aldrich RW, Lee AW (1993) Functional stoichiometry of Shaker potassium channel inactivation. Science 262:757–759
Madeja M, Müller V, Mußhoff U, Speckmann E-J (2000) Sensitivity of native and cloned hippocampal delayed rectifier potassium channels to verapamil. Neuropharmacology 39:202–210
Manganas LN, Trimmer JS (2000) Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem 275:29685–29693
Marin A, Lopez de Cerain A, Hamilton E, Lewis AD, Martinez-Penuela JM, Idoate MA, Bello J (1997) DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. Br J Cancer 76:923–929
Marom S, Levitan IB (1994) State-dependent inactivation of the Kv3 potassium channel. Biophys J 67:579–589
McGee R, Sansom MSP, Usherwood PNR (1988) Characterization of a delayed rectifier K+ channel in NG108–15 neuroblastoma×glioma cells: gating kinetics and the effects of enrichment of membrane phospholipids with arachodonic acid. J Membr Biol 102:21–34
Nilius B, Wohlrab W (1992) Potassium channels and regulation of proliferation of human melanoma cells. J Physiol (Lond) 445:537–548
Ouadid-Ahidouch H, Coppenolle FV, Bourhis XL, Belhaj A, Prevarskaya N (1999) Potassium channels in rat prostate epithelial cells. FEBS Lett 459:15–21
Parcej DN, Scott VE, Dolly JO (1992) Oligomeric properties of alpha-dendrotoxin-sensitive potassium ion channels purified from bovine brain. Biochemistry 31:11084–11088
Po S, Roberds S, Snyders DJ, Tamkun MM, Bennett PB (1993) Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circ Res 72:1326–1336
Rane SG (2000) The growth regulatory fibroblast IK channel is the predominant electrophysiological feature of rat prostatic cancer cells. Biochem Biophys Res Commun 269:457–463
Rasmusson RL, Zhang Y, Campbell DL, Comer MB, Castellino RC, Liu S, Strauss HC (1995) Bi-stable block by 4-aminopyridine of a transient K+ channel (Kv1.4) cloned from ferret ventricle and expressed in Xenopus oocytes. J Physiol (Lond) 485:59–71
Rauer H, Grissmer S (1996) Evidence for an internal phenylalkylamine action on the voltage-gated potassium channel Kv1.3. Mol Pharmacol 50:1625–1634
Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O (1994) Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369:289–294
Roeper J, Sewing S, Zhang Y, Sommer T, Wanner SG, Pongs O (1998) NIP domain prevents N-type inactivation in voltage-gated potassium channels. Nature 391:390–393
Ruppersberg JP, Schroter KH, Sakmann B, Stocker M, Sewing S, Pongs O (1990) Heteromultimeric channels formed by rat brain potassium channel proteins. Nature 345:535–537
Ruppersberg JP, Frank R, Pongs O, Stocker M (1991) Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature 353:657–660
Russell SN, Overturf KE, Horowitz B (1994) Heterotetramer formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. Am J Physiol 267:C1729–C1733
Scott VES, Muniz ZM, Sewing S, Lichtinghagen R, Parcej DN, Pongs O, Dolly JO (1994) Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of α-dendrotoxin-sensitive K+ channels in bovine brain. Biochem 33:1617–1623
Shamotienko OG, Parcej DN, Dolly JO (1997) Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochem 36:8195–8201
Skryma RN, Prevarskaya NB, Dufy-Barbe L, Odessa MF, Audin J, Dufy B (1997) Potassium conductance in the androgen-sensitive prostate cancer cell line, LNCaP: involvement in cell proliferation. Prostate 33:112–122
Smith P, Rhodes NP, Shortland AP, Fraser SP, Djamgoz MBA, Ke Y, Foster CS (1998) Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett 423:19–24
Spencer RH, Sokolov Y, Li H, Takenaka B, Milici AJ, Aiyar J, Nguyen A, Park H, Jap BK, Hall JE, Gutman GA, Chandy KG (1997) Purification, visualization, and biophysical characterization of Kv1.3 tetramers. J Biol Chem 272:2389–2395
Stühmer W, Ruppersberg JP, Schroter KH, Sakmann B, Stocker M, Giese KP, Perschke A, Baumann A, Pongs O (1989) Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J 8:3235–3244
Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA, Bennett C, Stein RB, Kaczmarek LK (1990) Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron 4:929–939
Acknowledgements
This work was supported by grants from the AICR (Association for International Cancer Research), KANCATAK and the MRC. We gratefully acknowledge the support of contributors to the Prostate Cancer Research Fund (PCRF). In addition, we thank Prof. James Trimmer for the Kv1.5 antibody, Prof. Clare Isacke for providing the secondary antibodies, Dr. N. Mohammed for help with preparation of the Western blot figure, and Dr. Jo Hirano for help with the PCR experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraser, S.P., Grimes, J.A., Diss, J.K.J. et al. Predominant expression of Kv1.3 voltage-gated K+ channel subunit in rat prostate cancer cell lines: electrophysiological, pharmacological and molecular characterisation. Pflugers Arch - Eur J Physiol 446, 559–571 (2003). https://doi.org/10.1007/s00424-003-1077-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-003-1077-0