Abstract
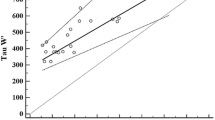
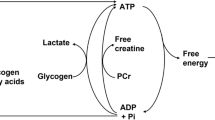
Whole-body O2 uptake (V̇O2), O2 deficit and the concentration of high-energy phosphates (determined by 31P spectroscopy) in human calf muscle were measured during moderate aerobic square-wave exercise of increasing intensity in ten volunteers. Net V̇O2 (above resting) increased linearly with mechanical power, yielding a delta efficiency of 13.1%. "Gross" O2 deficit increased linearly with net V̇O2. The fraction of phosphocreatine (PC) split at steady state increased linearly with the mechanical power and with the O2 deficit. If the [PC] in resting muscle is known, the slope of the regression between PC split and O2 deficit (in millimoles) yields the P/O2 ratio. To calculate this, the O2 deficit was corrected for the amount of O2 derived from the body stores, as obtained from literature data. The value so obtained, for a resting [PC] of 30 mM was 5.9, consistent with canonical textbook values. Furthermore, the ratio of "true" O2 deficit to steady-state V̇O2 is a measure of the time constant of V̇O2 kinetics at work onset at the muscle level: assuming a monoexponential time course without time delays it amounted to about 17 s, close to the value that can be expected in mammalian muscle at 37 °C.




Similar content being viewed by others
References
Arnold DL, Matthews PM, Radda GK (1984) Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med 1:307–315
Bangsbo J, Johansen L, Quistorff B, Saltin B (1993) NMR and analytical biochemical evaluation of CrP and nucleotides in the human calf during muscle contraction. J Appl Physiol 74:2034–2039
Barstow TJ, Buchtal S, Zanconato S, Cooper DM (1994) Muscle energetics and pulmonary uptake kinetics during moderate exercise. J Appl Physiol 77:1742–1749
Beaver WL, Lamarra N, Wasserman K (1981) Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51:1662–1675
Binzoni T, Ferretti G, Schenker K, Cerretelli P (1992) Phosphocreatine hydrolysis by 31P-NMR at the onset of constant-load exercise in humans. J Appl Physiol 73:1644–1649
Binzoni T, Hiltbrand E, Yano T, Cerretelli P (1997) Step vs. progressive exercise: the kinetics of phosphocreatine hydrolysis in human muscle. Acta Physiol Scand 159:209–215
Blei ML, Conley KE, Odderson IR, Esselman PC, Kushmerick MJ (1993) Individual variation in contractile cost and recovery in human skeletal muscle. Proc Natl Acad Sci USA 90:7396–7400
Boushel R, Langberg H, Green S, Skovgaard D, Bulow J, Kjar M (2000) Blood flow and oxygenation in peritendinous tissue and calf muscle during dynamic exercise in humans. J Physiol (Lond) 524:305–313
Buchli R, Meier D, Martin E, Boesiger P (1994) Assessment of Absolute Metabolite Concentrations in human tissue by 31P MRS in vivo. Part II: Muscle, liver, kidney. Magn Reson Med 32:453–458
Cautero M, Beltrami AP, di Prampero PE, Capelli C (2002) Breath-by-breath alveolar oxygen transfer at the onset of step exercise in humans: methodological implications. Eur J Appl Physiol 88:203–213
Cerretelli P, di Prampero PE (1987) Gas exchange in exercise. In: Fahri LE, Tenney SM (eds) Gas exchange (Handbook of Physiology series, Section 3, The respiratory system, vol. IV). American Physiology Society, Bethesda, pp 297–339
di Prampero PE (1981) Energetics of muscular exercise. Rev Physiol Biochem Pharmacol 89:143–210
di Prampero PE, Margaria R (1968) Relationship between O2 consumption, high energy phosphates and the kinetics of the O2 debt in exercise. Pflugers Arch 304:11–19
di Prampero PE, Davies CTM, Cerretelli P, Margaria R (1970) An analysis of O2 debt contracted in submaximal exercise. J Appl Physiol 29:547–551
di Prampero PE, Meyer M, Cerretelli P, Piiper J (1981) Energy sources and mechanical efficiency of anaerobic work in dog gastrocnemius. Pflugers Arch 389:257–262
Doyle VL, Payne GS, Collins DJ, Verrill MW, Leach MO (1997) Quantification of phosphorus metabolites in human calf muscle and soft-tissue tumours from localized MR spectra acquired using surface coils. Phys Med Biol 42:691–706
Francescato MP, Cettolo V (2001) A two pedal ergometer for in vivo MRS studies of human calf muscles. Magn Reson Med 46:1000–1005
Gaesser GA, Brooks GA (1975) Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol 38:1132–1139
Goudemant JF, Francaux M, Mottet I, Demeure R, Sibomana M, Sturbois X (1997) 31P NMR Saturation transfer study of the creatine kinase reaction in human skeletal muscle at rest and during exercise. Magn Reson Med 37:744–753
Grassi B (2000) Skeletal muscle VO2 on-kinetics: set by O2 delivery or by O2 utilization? Med Sci Sports Exerc 32:108–116
Harris RC, Hultman E, Nordesjo LO (1974) Glycogen, glycolitic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest: methods and variance of values. Scand J Clin Lab Invest 33:109–120
Iotti S, Frassinetti C, Zaniol P, Barbiroli B (1993) In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. NMR Biomed 6:248–253
Luhtanen P, Rahkila P, Rusko H, Viitasalo JT (1987) Mechanical work and efficiency in ergometer bicycling at aerobic and anaerobic thresholds. Acta Physiol Scand 131:331–337
Mader A (2002) Glycolysis and oxidative phosphorylation as a function of cytosolic phosphorylation state and power output of the muscle cell. Eur J Appl Physiol (In press) DOI 10.1007/s00421-002-0676-3
Masuda K, Choi JY, Shimojo H, Katsuta S (1999) Maintenance of myoglobin concentration in human skeletal muscle after heavy resistance training. Eur J Appl Physiol 79:347–352
McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT (1996) Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol 81:1331–1338
Molé PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T (1999) Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol 277:R173–R180
Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D (2001) Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comp Biol Med 31:269–286
Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D (2001) Java-based graphical user interface for the MRUI quantitation package. MAGMA 12:141–152
Piiper J, di Prampero PE, Cerretelli P (1968) Oxygen debt and high-energy phosphates in gastrocnemius muscle of the dog. Am J Physiol 215:523–531
Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ (1999) Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol (Lond) 518:921–932
Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ (2001) Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol (Lond) 537:291–303
Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ (2002) Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol (Lond) 541:991–1002
Roth K, Meyerhoff DJ, Naruse S, Gaber JR, Lawry TJ, Boska MD, Malsom GB, Welner MW (1989) Noninvasive quantitation of phosphorus metabolites in human tissue by NMR spectroscopy. J Magn Reson 81:299–311
Ryschon TW, Fowler MD, Arai AA, Wysong RE, Leighton SB, Clem TR, Balaban RS (1995) A multimode dynamometer for in vivo MRS studies of human skeletal muscle. J Appl Physiol 79:2139–2147
Schmid-Schoenbein G, Ross J Jr (1991) Structure-function relations in the peripheral circulation. In: West JB (ed) Best and Taylor's physiological basis of medical practice, 12th edn. Williams and Wilkins, Baltimore, p 119
Takahashi H, Inaki M, Fujimoto K, Katsuta S, Anno I, Niitsu M, Itai Y (1995) Control of the rate of phosphocreatine resynthesis after exercise in trained and untrained human quadriceps muscles. Eur J Appl Physiol 71:396–404
Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK (1983) Bioenergetics of intact human muscle: a 31P nuclear magnetic resonance study. Mol Biol Med 1:77–94
Walter G, Vandenborne K, Elliott M, Leigh JS (1999) In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Appl Physiol 519:901–910
Whipp BJ, Rossiter HB, Ward SA, Avery D, Doyle VL, Howe FA, Griffiths JR (1999) Simultaneous determination of muscle 31P and O2 uptake kinetics during whole body NMR spectroscopy. J Appl Physiol 86:742–747
Winter DA (1979) Biomechanics of human movement. Wiley-Liss, New York
Yoshida T, Watari H, Tagawa K (1996) Effects of active and passive recoveries on splitting of the inorganic phosphate peak determined by 31P-nuclear magnetic resonance spectroscopy. NMR Biomed 9:13–19
Acknowledgements
This research was supported by funds from the Ministero dell'Università e della Ricerca Scientifica (COFIN 1998, main investigator Prof. P.E. di Prampero).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
As a first approximation, during aerobic exercise of moderate intensity, the O2 uptake (V̇O2) at the onset of exercise is a monoexponential function of time:
where V̇O2 r and ΔV̇O2 s are the resting and the net steady-state V̇O2, respectively and τm is the time constant of the process, as measured at the mouth. The O2 deficit (grossO2 def) incurred at the onset of exercise can thus be calculated as:
Thus, the slope of the regression of ΔV̇O2 s on O2 deficit is the time constant of O2 uptake kinetics at the mouth. In all subjects, the individual regressions between grossO2 def and ΔV̇O2 s were characterized by r>0.980 (n=3) and the average τm was 41.8 s (see Fig. 3, solid dots). At the onset of moderate aerobic exercise below the anaerobic threshold, the grossO2 def is covered by the net O2 deficit, i.e. the amount of energy in O2 equivalents derived from PC splitting (O2 def), plus the amount of O2 derived from the body stores (ΔO2S), i.e.: grossO2 def=O2 def+ΔO2S. Thus, a prerequisite for the calculation of the net O2 deficit (O2 def) is knowledge of ΔO2S.
For all subjects and trials, we therefore estimated the changes of whole-body O2 stores. These comprise mainly the amounts of O2: i) contained in the lungs, ii) contained in the venous blood and iii) bound to muscle myoglobin [11, 12, 14]. Thus, the corresponding changes during the rest-to-work transients, or vice versa, yield the amount of O2 consumed by the muscles at work onset, but not "seen" at the mouth, or taken up at the mouth at work offset, but not consumed by the muscle.
The changes in the pulmonary oxygen stores (ΔO2Sp) can be estimated from the difference between the time constant of V̇O2 measured at the mouth (τm) and that measured at the alveolar level (τp):
According to McCreary et al. [26] the time constant of the O2 consumption adjustment at the onset of calf exercise, calculated at the alveolar level on a breath by breath basis, is 41.9 s (ignoring the highest value reported by those authors). However, according to Cautero et al. [10] the algorithms of Beaver et al. [4], used in [26], over-estimate the time constant of the alveolar kinetics by about 10 s. Thus, we assumed that the time course of V̇O2 at alveolar level was described by a monoexponential function with τp=31.9 s, a value essentially equal to the 34.3 s obtained at the alveolar level during cycloergometer exercise [10]. The individual ΔO2Sp values were then calculated from ΔV̇O2 s and the difference between the individual τm and τp=31.9 s and are reported in Table 2 for the three exercise conditions.
The changes in the venous blood stores (ΔO2Sv) can be estimated from the product of the venous blood volume (VBV) and the change between the arterio- to mixed venous blood oxygen difference at steady state: [Δ(C a−C v̄)s] and that prevailing at rest [Δ(C a−C v̄)r], i.e.:
where Q ̇s and Q ̇r are the cardiac outputs at steady-state during exercise and at rest, respectively. VBV was assumed to be 65% of the total blood volume, in turn assumed to represent (in litres) 7% of body mass (in kilograms) [36]. Q ̇ corresponding to the individual mechanical powers investigated in the present work was estimated from the data for calf exercises similar to the present ones [8]. This allowed us to determine the linear relationship between Q̇ (litres/minute) and mechanical power (Ws, watt) as: Q̇=0.40Ws+5.84 (n=5, r 2=0.945). Thus, the individual ΔO2Sv values were obtained from the appropriate V̇O2 and Q̇ values, the latter being derived from the corresponding mechanical power on the basis of the above equation. They are reported in Table 2 for the three exercise conditions.
The myoglobin desaturation for exercise intensities comparable to those of the present work was estimated from the data reported in [27]. These allowed us to calculate the relationship between percentage deoxymyoglobin (%deoxyMb) and mechanical power (Ws): %deoxyMb=2.09Ws+14.2 (n=4, r 2=0.968). Subsequently, knowing the individual mass of the plantar flexors (PF) and assuming a myoglobin concentration of 4.46 g/kg wet muscle [25] and a binding capacity of 1.34 mlO2/g myoglobin, it was possible to estimate the extra amount of oxygen released by myoglobin desaturation changes from rest to exercise (ΔO2Sm) as: ΔO2Sm=(2.09·Ws/100)·4.46·1.34·(PF mass). The net or true oxygen deficit was finally estimated for any given work load by subtracting the three changes in oxygen stores from the whole-body oxygen deficit: O2 def=grossO2 def−ΔO2Sp−ΔO2Sv−ΔO2Sm. The individual ΔO2Sm and O2 def values are also reported in Table 2 for the three exercise conditions.
Rights and permissions
About this article
Cite this article
Francescato, M.P., Cettolo, V. & di Prampero, P.E. Relationships between mechanical power, O2 consumption, O2 deficit and high-energy phosphates during calf exercise in humans. Pflugers Arch - Eur J Physiol 445, 622–628 (2003). https://doi.org/10.1007/s00424-002-0992-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-0992-9