Abstract
Purpose
Totally laparoscopic total gastrectomy (TLTG) is unpopular because reconstruction is difficult. In fact, esophagojejunostomy is the most difficult surgical technique in TLTG. We adopted functional end-to-end anastomosis for esophagojejunostomy to simplify the procedure. The present study assesses the feasibility and surgical outcomes of TLTG with functional end-to-end esophagojejunostomy.
Methods
We assessed the intraoperative and postoperative outcomes of 65 consecutive patients who underwent TLTG with functional end-to-end esophagojejunostomy at Tonan Hospital between January 2006 and August 2011.
Results
The mean surgical duration was 271.5 ± 64.7 min, and the mean blood loss was 85.2 ± 143.2 g. One patient (1.5 %) was converted to open surgery, and two patients (3.1 %) required reoperation due to ileus because of an internal hernia and jejunojejunostomy leakage. No reoperation was associated with functional end-to-end esophagojejunostomy. The mean hospital stay was 21.4 ± 13.5 days. Ten patients (15.4 %) developed postoperative complications, of which three (4.6 %) were anastomotic stenosis associated with functional end-to-end esophagojejunostomy. All of these were resolved by endoscopic dilation.
Conclusion
Functional end-to-end esophagojejunostomy in TLTG is safe and feasible.
Similar content being viewed by others
Introduction
Since the first laparoscopy-assisted distal gastrectomy for early gastric cancer was performed in 1991 [1], the development of dedicated instruments and surgical techniques has led to laparoscopic distal gastrectomy (LDG) to treat gastric cancer. However, laparoscopic total gastrectomy (LTG) for gastric cancer has not become as popular as LDG, because reconstruction is more complex after LTG than after LDG, especially for esophagojejunostomy. Construction of an esophagojejunostomy is technically difficult, and serious complications can arise. Use of a circular stapler can be technically challenging during laparoscopic procedures for obese patients and for those with a relatively narrow esophagus. Some surgeons have used an extracorporeal approach through a mini-laparotomy, constructing an end-to-side anastomosis with an end-to-end anastomosis stapler [2]. One recent report has described LTG using a transorally inserted anvil for esophagojejunostomy [3]. However, an optimal procedure for esophagojejunostomy has yet to be established.
The entire anastomotic procedure can be clearly viewed during totally LTG (TLTG), and thus, tension and damage can be prevented. We have performed 65 TLTG procedures using functional end-to-end anastomosis for esophagojejunostomy since January 2006. This article describes the intraoperative manipulations and postoperative outcomes of TLTG with functional end-to-end esophagojejunostomy.
Materials and methods
Patients
We enrolled 65 consecutive patients who underwent TLTG with functional end-to-end esophagojejunostomy at Tonan Hospital between January 2006 and August 2011. The eligibility criterion was clinical stage IA or IB gastric cancer that was preoperatively diagnosed by endoscopy, computed tomography, and endoscopic ultrasound. All of the patients provided written informed consent to participate in the study. Specimens were evaluated basically according to the Japanese Classification of Gastric Carcinoma established by the Japanese Research Society for Gastric Cancer [4].
Surgical technique
Patients are placed in the supine reverse Trendelenburg position with the legs apart under general anesthesia. Five Exel trocars (Ethicon Endo-Surgery, Cincinnati, OH, USA) are used, and a 12-mm paraumbilical port is subsequently extended to 3.0 cm when extracting specimens. After carbon dioxide pneumoperitoneum is achieved at a pressure of 10 mmHg, an electrolaparoscope (WA50013, Olympus Medical Systems, Tokyo, Japan) is introduced through this port, and the four other trocars (three 12 mm and one 5 mm) are positioned as shown in Fig. 1. We dissect lymph nodes and coagulated vessels using laparoscopic coagulation shears (SONOSURG-X, Olympus Medical Systems or Harmonic Ace, Ethicon Endo-Surgery). The basic extent of lymph node dissection in the present series was D1+, but patients with clinical N1 underwent D2+ splenectomy, both of which were achieved using TLTG [4].
Intracorporeal anastomosis with TLTG
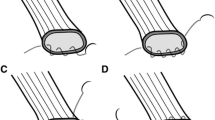
Figure 2 shows a functional end-to-end esophagojejunostomy in TLTG. The abdominal esophagus is exposed and transected using an Echelon 60 Endopath 60-mm linear stapler (Ethicon Endo-Surgery, Cincinnati, OH, USA) in the horizontal direction. Thereafter, 10-mm transverse incisions are created at the edges of the tip of the antimesenteric border between the jejunum and the right lateral wall of the abdominal esophagus. Both jaws of a 45-mm linear stapler (ETS Flex45, Ethicon Endo-Surgery, Cincinnati, OH, USA) are inserted into holes and fired. The entry hole for the Echelon 60 Endopath linear stapler is closed with one application of the stapler perpendicular to the first suture line. Functional end-to-end esophagojejunostomy is then accomplished. Some hand sutures are added for reinforcement, depending upon the conditions of the anastomosis. A side-to-side jejunostomy proceeds at a point 40 cm distal to the esophagojejunostomy using the ETS Flex45 linear stapler. The entry hole for this stapler is closed with a running suture (3–0 Vicryl, Ethicon). When we find a narrow esophageal hiatus that diminishes the working space during laparoscopic functional end-to-end esophagojejunostomy, we extend the hiatus to incise the diaphragm in the ventral direction because incising the esophageal crus to extend the hiatus can generate pneumothorax. We use a 45-mm linear stapler because we achieved functional end-to-end esophagojejunostomy using a 60-mm linear stapler during the early period of TLTG in three patients with anastomotic stenosis. The 60-mm linear stapler lacks grasping power, perhaps because the esophageal mucosa can slip off and become shortened before firing. Moreover, we occasionally found that the abdominal esophagus does not stretch during functional end-to-end esophagojejunostomy with a 60-mm linear stapler.
Clinicopathological findings
Information about the patients including gender, age, body mass index (BMI), operative duration, blood loss, number of lymph node dissections, pathological findings, and perioperative complications was collected from the medical and anesthesia records.
All values are expressed as means ± standard deviation.
Results
Table 1 shows the clinical features of the patients. The mean age was 65.9 ± 10.2 years, and 45 (69.2 %) male and 20 (30.8 %) female patients underwent TLTG with functional end-to-end esophagojejunostomy. Their mean BMI was 23.5 ± 4.0 kg/m2. The upper and middle regions of the stomach were affected by malignancies in 53 (81.5 %) and 12 (18.5 %) patients, respectively. The clinical stages were stages IA and IB in 41 (63.1 %) and 24 (36.9 %) patients, respectively. Table 2 shows details of the surgical and pathological data. The mean surgical duration was 271.5 ± 64.7 min, and the mean blood loss was 85.2 ± 143.2 g. One (1.5 %) patient was converted to open surgery due to low blood pressure of uncertain cause during the procedure. The type of lymph node dissection was D1+ in 62 (95.4 %) patients and D2+ splenectomy in 3 (4.6 %) patients, and 30.2 ± 12.4 lymph nodes were dissected. The cancer had invaded the mucosa, submucosa, muscle, subserosa, and serosa in 18 (27.7 %), 28 (43.1 %), 9 (13.8 %), 5 (7.7 %), and 5 (7.7 %) patients, respectively. The histological type was well, moderately, and poorly differentiated in 19 (29.2 %), 16 (24.6 %), and 30 (46.2 %) patients, respectively. Twelve (18.5 %) patients had lymph node metastasis. Table 3 shows the postoperative outcomes. Ten (15.4 %) patients developed postoperative complications comprising jejunojejunostomy leakage in one (1.5 %), duodenal stump leakage in one (1.5 %), esophagojejunostomy stenosis in three (4.6 %), pancreatic juice fistula in two (3.1 %), paralytic ileus in one (1.5 %), and wound infection in two (3.1 %). No esophagojejunostomy leaked after TLDG, and related complications that developed in three (4.6 %) patients were resolved by endoscopic dilation. One patient who underwent reoperation for afferent loop syndrome died of septic shock on postoperative day 90. The means of elapsed time until first flatus, resuming a soft diet, and discharge were 1.9 ± 0.7, 4.6 ± 1.8, and 21.4 ± 13.5 days, respectively. Cancer did not recur in any of the patients during a median follow-up of 37 (11–68) months.
Discussion
Laparoscopic surgery confers upon patients the advantages of faster recovery, fewer complications, reduced hemorrhaging that reduces the likelihood of needing a blood transfusion, a smaller incision that reduces pain, the probability of intestinal obstruction, and the risk of wounding. Since its introduction in 1994, LDG for gastric cancer has become a commonplace due to the development of dedicated instruments and surgical techniques [1]. Many surgeons have described their experiences with totally laparoscopic gastrectomy and have found it safe and feasible [5–8]. Kim et al. concluded that TLDG helps to improve early surgical outcomes [9]. A policy of totally laparoscopic gastrectomy was adopted at our hospital from its inception because we considered that it would confer several advantages. Extracorporeal anastomosis via mini-laparotomy incisions during laparoscopy-assisted gastrectomy could cause forceful tension and damage structures around an anastomosis because of limited vision, especially in obese patients [6, 10]. The entire anastomotic procedure can be clearly viewed during totally laparoscopic gastrectomy, which prevents such tension and damage. However, circular staplers are usually used for esophagojejunostomy in most LTG with mini-laparotomy incisions [11, 12]. The intra-abdominal application of a circular stapler might be challenging, and intracorporeal purse-string sutures and anvil placement are required for obese patients as well as for those with a relatively narrow esophagus [11, 13]. Okabe et al. noted that a circular stapler has not been specifically designed for endoscopic surgery and such staplers have usually been extracorporeally applied because of difficulties with applying them under a limited laparoscopic view [14]. If force is applied to a relatively large anvil, the mucosa can slip off and rupture the esophageal wall, which would result in serious complications [15]. In contrast, a linear stapler can be easily manipulated intra-abdominally. Steichen introduced a functional end-to-end anastomosis that has been adopted worldwide [16]. Matsui et al. and Lee et al. concluded that functional end-to-end esophagojejunostomy after a total gastrectomy is convenient, safe, reliable, and independent of the esophagus and depth of the esophageal hiatus [17, 18]. Functional end-to-end esophagojejunostomy after total gastrectomy in open surgery has become accepted, whereas laparoscopic esophagojejunostomy seems quite rare. We adopted this technique from the introduction of TLTG at our hospital. We have not experienced esophagojejunostomy leakage, and esophagojejunostomy stenosis that developed in three (4.6 %) patients was resolved by endoscopic dilation, the frequency of which varies from 3 to 10 % when a circular stapler is applied [19, 20]. One of our patients died in the hospital due to afferent loop syndrome caused by an internal hernia, which is a potentially serious complication after TLTG. Because of this experience, we close mesenteric defects using an intracorporeal suture. We do not apply this method to patients with tumors that have invaded the esophagus, and we perform intracorporeal circular stapling for esophagojejunostomy using a transorally inserted OrVilTM anvil (Covidien, Mansfield, MA, USA). If a tumor is close to the esophago-cardiac junction, we transect the abdominal esophagus before starting an esophagojejunostomy. We have not experienced anastomotic stenosis since using the 45-mm linear stapler, which has more grasping power. As a result, the suture line between the esophageal and the intestinal wall does not shift. The long-term outcomes of functional end-to-end esophagojejunostomy in TLTG remain unknown, and thus, further clinical trials and prospective controlled studies are required for comparisons with other types of anastomosis. We achieved functional end-to-end esophagojejunostomy in TLTG without anastomotic leakage and consider this technique safe and feasible.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Asao T, Hosouchi Y, Nakabayashi T, Haga N, Mochiki E, Kuwano H (2001) Laparoscopically assisted total or distal gastrectomy with lymph node dissection for early gastric cancer. Br J Surg 88:128–132
Jeong O, Park YK (2009) Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVilTM) after laparoscopic total gastrectomy. Surg Endosc 23:2624–2630
Japanese Gastric Cancer Association (1998) Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1:10–24
Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M (2002) Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy. J Am Coll Surg 195:284–287
Kim JJ, Song KY, Chin HM, Kim W, Jeon HM, Park CH, Park SM (2008) Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc 22:436–442
Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, Chin HM, Hur H (2008) Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg 12:1015–1021
Tanimura S, Higashino M, Fukunaga Y, Takemura M, Nishikawa T, Tanaka Y, Fujiwara Y, Osugi H (2008) Intracorporeal Billroth I reconstruction by triangulating stapling technique after laparoscopic distal gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech 18:54–58
Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS (2011) A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc 25:1076–1082
Choi YY, Kim YJ (2011) Intracorporeal anastomosis using a Lapra-ty clip in laparoscopic distal gastrectomy: initial clinical experiences. J Laparoendosc Adv Surg Tech A 21:51–55
Dulucq J, Wintringer P, Perissat J, Mahajna A (2005) Completely laparoscopic total and partial gastrectomy for benign and malignant diseases: a single institute’s prospective analysis. J Am Coll Surg 200:191–197
Usui S, Yoshida T, Ito K, Hiranuma S, Kudo S, Iwai T (2005) Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech 15:309–314
Ziqiang W, ZhiMin C, Jun C, Xiao L, Huaxing L, PeiWu Y (2008) A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc 22:2091–2094
Okabe H, Obama K, Tanaka E, Nomura A, Kawamura J, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y (2009) Intracorporeal esophagojejunal anastomosis after gastrectomy for patients with gastric cancer. Surg Endosc 23:2167–2171
Okushiba S, Kawarada Y, Shichinohe T, Manase H, Kitashiro S, Katoh H (2005) Esophageal delta-shaped anastomosis: a new method of stapled anastomosis for the cervical esophagus and digestive tract. Surg Today 35:341–344
Steichen FM (1968) The use of staplers in anatomical side-to-side and functional end-to-end enteroanastomoses. Surgery 64:948–953
Matsui H, Uyama I, Sugioka A, Fujita J, Komori Y, Ochiai M, Hasumi A (2002) Linear stapling forms improved anastomoses during esophagojejunostomy after a total gastrectomy. Am J Surg 184:58–60
Lee IS, Kim TH, Kim KC, Yook JH, Kim BS (2012) Modified techniques and early outcomes of totally laparoscopic total gastrectomy with side-to-side esophagojejunostomy. J Laparoendosc Adv Surg Tech A 22:876–880
Dorsey JS, Esses S, Goldberg M, Stone R (1980) Esophagogastrectomy using the auto suture EEA surgical stapling instrument. Ann Thorac Surg 30:308–312
Wong J, Cheung H, Lui R, Fan YW, Smith A, Siu KF (1987) Esophagogastric anastomosis performed with a stapler: the occurrence of leakage and stricture. Surgery 101:408–415
Conflicts of interest
All authors state that they have no commercial associations that might pose a conflict in connection with the submitted article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ebihara, Y., Okushiba, S., Kawarada, Y. et al. Outcome of functional end-to-end esophagojejunostomy in totally laparoscopic total gastrectomy. Langenbecks Arch Surg 398, 475–479 (2013). https://doi.org/10.1007/s00423-013-1051-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-013-1051-z