Abstract
Background
For management of thyroid nodules, distinction between benign and malignant tumours is essential. The study was performed to evaluate the diagnostic value of molecular markers in different thyroid tumours.
Materials and methods
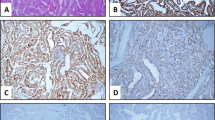
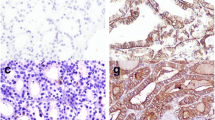
Immunohistochemistry for CD56, HBME-1, COX-2, Ki-67, p53 and E-cadherin (E-CAD) was performed in 113 benign and 35 malignant thyroid lesions including 36 follicular adenomas (FA), 77 colloid goitres, 26 papillary thyroid carcinomas (PTC) and 9 follicular carcinomas (FC). The results were scored semiquantitatively by staining intensity (0–3 scale) and percentage of positive cells.
Results
PTC was characterised by decreased E-CAD and CD56 expression in contrast to surrounding benign thyroid tissues. HBME-1 expression was absent in benign thyroid tissues but was notably high in PTC and occasionally in FC. The expression of E-CAD and CD56 in FA was significantly higher than in the surrounding thyroid tissues. No expression of p53 was found in any group. The expression of COX-2 was low in all lesions. The proliferation activity by Ki-67 was generally low; however, it was significantly higher in cancers.
Conclusions
The panel consisting of three markers, HBME-1, E-CAD and CD56, can be recommended as an adjunct to morphology criteria. HBME-1 is found in malignant lesions only and is the most sensitive and specific single marker in PTC. Decreased expression of E-CAD and CD56 distinguishes PTC from FA and FC. Both FA and FC are characterised by high expression of E-CAD and CD56. The practical use of Ki-67 is difficult due to low values. The role of adhesion factors in thyroid malignancies may be superior in comparison with cell proliferation.





Similar content being viewed by others
References
Gharib H, Papini E (2007) Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin N Am 36:707–735
Topliss D (2004) Thyroid incidentaloma: the ignorant in pursuit of the impalpable. Clin Endocrinol (Oxf) 60:18–20
Hundahl SA et al (1998) A National Cancer Data Base report on 53, 856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648
Finley DJ et al (2004) Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. J Clin Endocrinol Metab 89:3214–3223
Liu YY et al (2008) Combined immunostaining with galectin-3, fibronectin-1, CITED-1, Hector Battifora mesothelial-1, cytokeratin-19, peroxisome proliferator-activated receptor-{gamma}, and sodium/iodide symporter antibodies for the differential diagnosis of non-medullary thyroid carcinoma. Eur J Endocrinol 158:375–384
Haugen BR, Woodmansee WW, McDermott MT (2002) Towards improving the utility of fine-needle aspiration biopsy for the diagnosis of thyroid tumours. Clin Endocrinol (Oxf) 56:281–290
Bancroft JD, Gamble M (2003) Theory and practice of histological techniques, international edition. Churchill Livingstone, Edinburgh
DeLellis RA (2006) Pathology and genetics of thyroid carcinoma. J Surg Oncol 94:662–669
Altman DG, Machin D, Bryant TN, Gardner MJ (2005) Statistics with confidence, 2nd edn. BMJ, Bristol
Sheibani K et al (1992) Immunopathologic and molecular studies as an aid to the diagnosis of malignant mesothelioma. Hum Pathol 23:107–116
Saleh HA et al (2010) Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol 5:9
Miettinen M, Karkkainen P (1996) Differential reactivity of HBME-1 and CD15 antibodies in benign and malignant thyroid tumours. Preferential reactivity malignant tumours. Virchows Arch 429:213–219
Sack MJ et al (1997) HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma. Mod Pathol 10:668–674
Mai KT et al (2002) Reduced HBME-1 immunoreactivity of papillary thyroid carcinoma and papillary thyroid carcinoma-related neoplastic lesions with Hurthle cell and/or apocrine-like changes. Histopathology 40:133–142
Ito Y et al (2005) HBME-1 expression in follicular tumor of the thyroid: an investigation of whether it can be used as a marker to diagnose follicular carcinoma. Anticancer Res 25:179–182
Papotti M et al (2005) Galectin-3 and HBME-1 expression in well-differentiated thyroid tumors with follicular architecture of uncertain malignant potential. Mod Pathol 18:541–546
Prasad ML et al (2005) Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol 18:48–57
Rossi ED et al (2006) Simultaneous immunohistochemical expression of HBME-1 and galectin-3 differentiates papillary carcinomas from hyperfunctioning lesions of the thyroid. Histopathology 48:795–800
Saggiorato E et al (2005) Characterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical application. Endocr Relat Cancer 12:305–317
de Micco et al (2008) Utility of malignancy markers in fine-needle aspiration cytology of thyroid nodules: comparison of Hector Battifora mesothelial antigen-1, thyroid peroxidase and dipeptidyl aminopeptidase IV. Br J Cancer 98:818–823
Nasr MR et al (2006) Immunohistochemical markers in diagnosis of papillary thyroid carcinoma: utility of HBME1 combined with CK19 immunostaining. Mod Pathol 19:1631–1637
Saleh HA et al (2009) Differential expression of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsy. Cytojournal 6:18
Nga ME et al (2008) HBME-1 and CK19 are highly discriminatory in the cytological diagnosis of papillary thyroid carcinoma. Diagn Cytopathol 36:550–556
Scognamiglio T et al (2006) Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol 126:700–708
Mase T et al (2003) HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J 50:173–177
Park YJ et al (2007) Diagnostic value of galectin-3, HBME-1, cytokeratin 19, high molecular weight cytokeratin, cyclin D1 and p27(kip1) in the differential diagnosis of thyroid nodules. J Korean Med Sci 22:621–628
Zeromski J et al (1992) Expression of CD56 (NKH-1) differentiation antigen in human thyroid epithelium. Clin Exp Immunol 89:474–478
El Demellawy D, Nasr A, Alowami S (2009) An updated review on the clinicopathologic aspects of arrhythmogenic right ventricular cardiomyopathy. Am J Forensic Med Pathol 30:78–83
El Demellawy D, Nasr A, Alowami S (2008) Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol 3:5
Scarpino S et al (2007) Papillary carcinoma of the thyroid: low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J Pathol 212:411–419
Hirohashi S, Kanai Y (2003) Cell adhesion system and human cancer morphogenesis. Cancer Sci 94:575–581
Eidelman S et al (1989) Expression of the cell-cell adhesion glycoprotein cell-CAM 120/80 in normal human tissues and tumors. Am J Pathol 135:101–110
Brabant G et al (1993) E-cadherin: a differentiation marker in thyroid malignancies. Cancer Res 53:4987–4993
Soares P et al (1997) E-cadherin gene alterations are rare events in thyroid tumors. Int J Cancer 70:32–38
von Wasielewski R et al (1997) Immunohistochemical detection of E-cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Cancer Res 57:2501–2507
Graff JR et al (1998) Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle's cell, and poorly differentiated human thyroid carcinoma. Cancer Res 58:2063–2066
Choi YL et al (2005) Immunoexpression of HBME-1, high molecular weight cytokeratin, cytokeratin 19, thyroid transcription factor-1, and E-cadherin in thyroid carcinomas. J Korean Med Sci 20:853–859
Smyth P et al (2001) Real-time quantitative analysis of E-cadherin expression in ret/PTC-1-activated thyroid neoplasms. Int J Surg Pathol 9:265–272
Fosslien E (2000) Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci 30:3–21
Ito Y et al (2003) Cyclooxygenase-2 expression in thyroid neoplasms. Histopathology 42:492–497
Specht MC et al (2002) Cyclooxygenase-2 expression in thyroid nodules. J Clin Endocrinol Metab 87:358–363
Casey MB et al (2004) Expression of cyclooxygenase-2 and thromboxane synthase in non-neoplastic and neoplastic thyroid lesions. Endocr Pathol 15:107–116
Cornetta AJ et al (2002) Cyclooxygenase-2 expression in human thyroid carcinoma and Hashimoto's thyroiditis. Laryngoscope 112:238–242
Kim SJ et al (2003) Immunohistochemical expression of COX-2 in thyroid nodules. Korean J Intern Med 18:225–229
Nose F et al (2002) Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors: significant correlation with inducible nitric oxide synthase. Am J Clin Pathol 117:546–551
Haynik DM, Prayson RA (2005) Immunohistochemical expression of cyclooxygenase 2 in follicular carcinomas of the thyroid. Arch Pathol Lab Med 129:736–741
Saad AG et al (2006) Proliferative activity of human thyroid cells in various age groups and its correlation with the risk of thyroid cancer after radiation exposure. J Clin Endocrinol Metab 91:2672–2677
Ziad el A et al (2008) Immunoexpression of TTF-1 and Ki-67 in a coexistent anaplastic and follicular thyroid cancer with rare long-life surviving. Folia Histochem Cytobiol 46:461–464
Novosel I et al (2006) p53, bcl-2 and Ki-67 in the diagnosis of insular thyroid gland cancer. Case report with a review of literature. Lijec Vjesn 128:264–267
Akslen LA, Varhaug JE (1995) Oncoproteins and tumor progression in papillary thyroid carcinoma: presence of epidermal growth factor receptor, c-erbB-2 protein, estrogen receptor related protein, p21-ras protein, and proliferation indicators in relation to tumor recurrences and patient survival. Cancer 76:1643–1654
Martinez J, Georgoff I, Levine AJ (1991) Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev 5:151–159
Finlay CA, Hinds PW, Levine AJ (1989) The p53 proto-oncogene can act as a suppressor of transformation. Cell 57:1083–1093
Morita N, Ikeda Y, Takami H (2008) Clinical significance of p53 protein expression in papillary thyroid carcinoma. World J Surg 32:2617–2622
Dobashi Y et al (1993) Overexpression of p53 as a possible prognostic factor in human thyroid carcinoma. Am J Surg Pathol 17:375–381
Chen BK et al (1999) Co-overexpression of p53 protein and epidermal growth factor receptor in human papillary thyroid carcinomas correlated with lymph node metastasis, tumor size and clinicopathologic stage. Int J Oncol 15:893–898
Kobayashi T et al (1995) Clinicopathological findings and p53 expression of thyroid cancer in children. Surg Today 25:217–221
Acknowledgment
The study was supported by grant 2009/0147/1DP/1.1.2.1.2/09/IPIA/VIAA/009 from European Social Fund.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozolins, A., Narbuts, Z., Strumfa, I. et al. Diagnostic utility of immunohistochemical panel in various thyroid pathologies. Langenbecks Arch Surg 395, 885–891 (2010). https://doi.org/10.1007/s00423-010-0690-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-010-0690-6