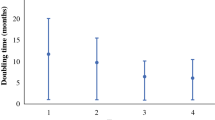
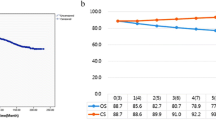
Abstract
Background and aims
Recurrence is the main reason for early death of cancer patients. Therefore, survival curves are regarded as crucial tools for clinicians to evaluate therapies and to estimate the patients’ prognosis. Current models for the development of recurrence are based on the assumption of a residual cancer cell burden after therapy. Accordingly, in assumption of an exponential cell growth of the cancer cells an S-shaped decline of the survival curve is expected with earlier onset in case of advanced cancer and a later manifestation in case of little residual tumour. However, many survival curves do not reflect any S-shaped configuration and thus may question the current concept.
Materials and methods
To test, whether the incidence for developing a recurrence may be considered as remaining constant over time, we analysed survival data of 446 patients with gastric cancer, operated from 1975–2001 in the Surgical Department of the RWTH Aachen.
Results
All survival curves, even after sub-grouping according to UICC stage, show a monotonous decline without any apparent S-shape. The impact of TNM and UICC stage to predict the survival in patients is estimated by Cox regression, for estimation the risk for death a logistic regression is performed. Whereas the presence of metastasis lowers the prognosis significantly with a hazard ratio of 1.57 and an odds ratio of 7.56, respectively, a significant relevance for the UICC stage, the tumour size or the lymph node status cannot be proven. Furthermore, the two assumptions: (1) that 20% of patients who are still alive after 5 years have been cured, and (2) that the remainder develop a recurrence in constantly 7.3% per month, are able to configure the survival curve almost precisely (correlation coefficient between calculated and observed survival rate r > 0.99).
Conclusion
The absence of any S-shaped survival curve configuration is not in accordance with the focus on residual tumour clones with its exponential growth as the decisive process for recurrence development. In contrast, the monotonous decline of surviving patients is best reflected by a constant incidence of recurrence. The (time) constancy of the recurrence incidence encourages the view of recurrent cancer as a chronic problem of a carcinogenic environment. Furthermore, it supports new anticancer therapies which rather targets cell regulation and immunology instead of acting cytotoxic.



Similar content being viewed by others
References
Kern SE (2002) Whose hypothesis? Ciphering, sectorials, D lesions, freckles and the operation of Stigler’s Law. Cancer Biol Ther 1:571–581
Lichtenstein AV (2005) On evolutionary origin of cancer. Cancer Cell Int 5:5
D'Angelica M, Gonen M, Brennan MF et al (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240:808–816
Igney F (2002) Tumour counterattack apoptose-vermittelte immunsuppression durch tumoure. http://elib.uni-stuttgart.de/opus/volltexte/2003/1263; dissertation at the faculty of geo- and biological sciences of the university of Stuttgart
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumour cell migration, invasion, and metastasis. Cell 124:263–266
Brabletz T, Jung A, Spaderna S et al (2005) Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer 5:744–749
Lichtenstein AV (2005) Cancer as a programmed death of an organism. Biochemistry (Mosc) 70:1055–1064
Maffini MV, Calabro JM, Soto AM, Sonnenschein C (2005) Stromal regulation of neoplastic development: age-dependent normalization of neoplastic mammary cells by mammary stroma. Am J Pathol 167:1405–1410
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458
Slaughter DP, Southwick HW, Smejkal W (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6:963–968
Braakhuis BJ, Tabor MP, Kummer JA et al (2003) A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res 63:1727–1730
Marrelli D, De Stefano A, de Manzoni G et al (2005) Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg 241:247–255
Jung H, Beck-Bornholdt HP, Svoboda V et al (2001) Quantification of late complications after radiation therapy. Radiother Oncol 61:233–246
Bennett JJ, Gonen M, D’Angelica M et al (2005) Is detection of asymptomatic recurrence after curative resection associated with improved survival in patients with gastric cancer? J Am Coll Surg 201:503–510
Garcia SB, Park HS, Novelli M, Wright NA (1999) Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol 187:61–81
Aractingi S, Kanitakis J, Euvrard S et al (2005) Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res 65:1755–1760
Schmeichel KL, Weaver VM, Bissell MJ (1998) Structural cues from the tissue microenvironment are essential determinants of the human mammary epithelial cell phenotype. J Mammary Gland Biol Neoplasia 3:201–213
Rubio D, Garcia-Castro J, Martin MC et al (2005) Spontaneous human adult stem cell transformation. Cancer Res 65:3035–3039
Grizzi F, Di Ieva A, Russo C et al (2006) Cancer initiation and progression: an unsimplifiable complexity. Theor Biol Med Model 3:37
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft, SFB 542 project C4. We gratefully acknowledge the contributions of Michael Sarr, Mayo Clinic Rochester, for critical discussions and advice as well as editorial support with the manuscript. Furthermore, we are grateful to Marc Jansen for sharing clinical data of the patient cohort.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klinge, U., Ackermann, D., Lynen-Jansen, P. et al. The risk to develop a recurrence of a gastric cancer—is it independent of time?. Langenbecks Arch Surg 393, 149–155 (2008). https://doi.org/10.1007/s00423-007-0272-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-007-0272-4