Abstract
Background
Muscles are the primary contributors to joint loading. Loading is typically associated with the onset and progression of joint degeneration, and in turn, joint degeneration is known to affect negatively the control of muscle forces and co-ordination patterns. Nevertheless, the role of muscles in joint adaptation and degeneration has been largely ignored. Here, we review some of our research on the in vivo changes in muscular forces and joint loading in animal models of osteoarthritis and in patients with joint injury and disease. We attempt to emphasize the close dependence of muscle forces, joint loading and degeneration and, vice versa, try to point out how joint degeneration affects muscle forces and joint loading.
Material and methods
We measured the forces and electromyographic signals in normal and anterior cruciate ligament transected feline knees and measured (1) a consistent decrease in the knee extensor and ankle extensor muscle forces for weeks following intervention; (2) a corresponding decrease in the static and dynamic external ground reaction forces; and (3) a change in the electromyographic signals (in terms of the firing patterns of individual muscles and of the co-ordination of extensors and flexors during locomotion). We introduced results on the biosynthetic response of articular cartilage to controlled, in vivo, loading and discuss preliminary results from an experimental animal model of muscle weakness. In contrast to much of the published literature, loading, in our case, is introduced by controlled nerve stimulation and the corresponding muscular forces that load the joint in its in vivo configuration.
Results
We found that short-term loading (30–60 min) in the cat knee produces distinct up-regulation of mRNA of specific metalloproteinases (MMPs) and some of the MMP inhibitors. In our newly developed muscle-weakness model, we confirmed that controlled Botox injections in the rabbit knee extensor muscles cause a 60–80% decrease in muscle force, and that these changes in muscle force are associated with changes in the external ground reaction forces, and most importantly, that muscle weakness seems to be associated with degeneration of the knee in the absence of joint instability or any other intervention.
Conclusion
From the results of our research, we conclude that muscle health and muscle rehabilitation are key components for the successful prevention of, and recovery from, joint injury and disease.
















Similar content being viewed by others
References
Taber LA (1995) Biomechanics of growth, remodeling, and morphogenesis. Appl Mech Rev 48:487–545
Carter DR (1987) Mechanical loading history and skeletal biology. J Biomech 20:1095–1099
Cowin SC (1986) Wolff's law of trabecular architecture at remodeling equilibrium. J Biomech Eng 108:83–88
Goldstein SA, Matthews LS, Kuhn JL, Hollister SJ (1991) Trabecular bone remodeling: an experimental model. J Biomech 24[Suppl 1]:135–150
Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B, Sloof TJ (1987) Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech 20:1135–1150
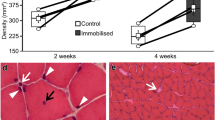
Booth FW (1982) Effect of limb immobilization on skeletal muscle. J Appl Physiol 52:1113–1118
Goldberg AL, Etlinger JD, Goldspink DF, Jablecki C (1975) Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports Exerc 7:248–261
Simard CP, Spector SA, Edgerton VR (1982) Contractile properties of rat hind limb muscles immobilized at different lengths. Exp Neurol 77:467–482
Tabary JC, Tabary C, Tarieu C, Tarieu G, Goldspink G (1972) Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 224:231–244
Vandenburgh HH (1982) Dynamic mechanical orientation of skeletal myofibers in vitro. Dev Biol 93:438–443
Adams ME (1989) Cartilage hypertrophy following canine anterior cruciate ligament transection differs among different areas of the joint. J Rheumatol 16:818–824
Brandt KD, Braunstein EM, Visco DM, O'Connor B, Heck D, Albrecht M (1991) Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol 18:436–446
Jurvelin J, Kiviranta F, Tammi M, Helminen HJ (1986) Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop 207:246–252
Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS (1994) Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res 12:451–463
Pond MJ, Nuki G (1973) Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis 32:387–388
McDevitt C, Gilbertson E, Muir H (1977) An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br 59:24–35
Moskowitz RW, Howell DS, Goldberg VM (1979) Cartilage proteoglycan alterations in an experimentally induced model of rabbit osteoarthritis. Arthritis Rheum 22:155–163
Bandi W (1972) Chondromalacia patellae und femoro-patellare Arthrose. Aetiologie, Klinik, und Therapie. Helv Chir Acta Suppl 11:3–70
Insall J, Goldberg V, Salvati E (1972) Recurrent dislocation and the high-riding patella. Clin Orthop 88:67–69
Outerbridge RE, Dunlop JAY (1975) The problem of chondromalacia. Clin Orthop 110:177–196
Maquet PGJ (1979) Mechanics and osteoarthritis of the patellofemoral joint. Clin Orthop 144:70–73
Kettlekamp DB, Coyler RA (1984) Osteoarthritis of the knee. In: Moskowitz RW, Howell DS (eds) Osteoarthritis: diagnosis and management. Saunders, Philadelphia, pp 403–421
Mankin HJ, Brandt KD, Shulman LE (1986) Workshop on the pathogenesis of osteoarthritis: proceedings and recommendations. J Rheumatol 13:1127–1160
Wong M, Wuethrich P, Eggli P, Hunziker E (1996) Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res 14:424–432
Wong M, Wuethrich P, Buschmann MD, Eggli P, Hunziker E (1997) Chondrocyte biosynthesis correlates with local tissue strain in statically compressed adult articular cartilage. J Orthop Res 15:189–196
Quinn TM, Grodzinsky AJ, Hunziker EG, Sandy JD (1998) Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res 16:490–499
Andrews JG (1974) Biomechanical analysis of human motion. Kinesiology 4:32–42
Clark A, Herzog W, Matyas JR, Barclay L, Leonard TR (2001) Proceedings of the 18th Congress of the International Society of Biomechanics, pp 162–163
Suter E, Herzog W, Leonard TR, Nguyen H. 1998. One-year changes in hindlimb kinematics, ground reaction forces and knee stability in an experimental model of osteoarthritis. J Biomech 31:511–517
Brandt KD, Myers SL, Burr D, Albrecht M (1991) Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum 34:1560–1570
Vilensky JA, O'Connor BL, Brandt KD, Dunn EA, Rogers PI, DeLong CA (1994) Serial kinematic analysis of the unstable knee after transection of the anterior cruciate ligament: temporal and angular changes in a canine model of osteoarthritis. J Orthop Res 12:229–237
O'Connor BL, Visco DM, Heck D, Myers SL, Brandt KD (1989) Gait alterations in dogs after transection of the anterior cruciate ligament. Arthritis Rheum 32:1142–1147
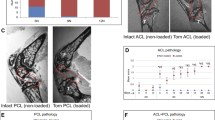
Hasler EM, Herzog W, Leonard TR, Stano A, and Nguyen H (1998) In-vivo knee joint loading and kinematics before and after ACL transection in an animal model. J Biomech 31:253–262
Hurley MV, Jones DW, Newham DJ (1994) Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Colch) 86:305–310
Salo PT, Seeratten RA, Erwin WM, Bray RC (2002) Evidence for a neuropathic contribution to the development of spontaneous knee osteoarthritis in a mouse model. Acta Orthop Scand 73:77–84
Visco DM, O'Connor BL, Heck D (1990) ORS meeting 1990, 36:559
Brandt KD (1997) Putting muscle into osteoarthritis. Ann Intern Med 127:154–155
Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinsky FD (1997) Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med 127:97–104
Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca S, Braunstein EM, Byrd D (1998) Reduced quadriceps strength relative to body weight. A risk factor for knee osteoarthritis in women? Arthritis Rheum 41:1951–1959
Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B (1990) Aging alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140:55–62
Inokuchi S, Ishikawa H, Iwamoto S, Kimura T (1975) Age-related changes in the histological composition of the rectus abdominis muscle of the adult human. Hum Biol 47:231–249
Overend TJ, Cunningham DA, Paterson DH, Lefcoe MS (1992) Thigh composition in young and elderly men determined by computed tomography. Clin Physiol 12:629–640
Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M (1983) Distribution of different fibre types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6:588–595
McComas AJ (1996) Skeletal muscle: form and function. Human Kinetics, Champaign, Illinois.
Campbell MJ, McComas AJ, Petito F (1973) Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36:174–182
Krebs DE, Elbaum L, Riley PO, Hodge WA, Mann RW (1991) Exercise and gait affects on in vivo hip contact pressures. Phys Ther 71:301–309
Strickland EM, Fares M, Krebs DE, Riley PO, Givens-Heiss DL, Hodge WA, Mann RW (1992) In vivo acetabular contact pressures during rehabilitation. I. Acute phase. Phys Ther 72:691–699
Givens-Heiss DL, Krebs DE, Riley PO, Strickland EM, Fares M, Hodge WA, Mann RW (1992) In vivo acetabular contact pressures during rehabilitation. II. Postacute phase. Phys Ther 72:700–705
Fagerson TL, Krebs DE, Harris BA, Mann RW (1995) Examining shibboleths of hip rehabilitation protocols using in vivo contact pressures from an instrumented hemiarthroplasty. Physiotherapy (London) 81:533–540
Park SS, Krebs DE, Mann RW (1999) Hip muscle co-contraction: evidence from concurrent in vivo pressure measurement and force estimation. Gait Posture 10:211–222
Kotzar GM, Davy DT, Goldberg VM, Heiple KG, Berilla J, Heiple G, Brown RH, Burstein AH (1991) Telemeterized in vivo hip joint force data: a report on two patients after total hip surgery. J Orthop Res 9:621–633
Bergmann G, Graichen F, Rohlmann A (1993) Hip joint loading during walking and running, measured in two patients. J Biomech 26:969–990
Herzog W, Leonard TR (1991) Validation of optimization models that estimate the forces exerted by synergistic muscles. J Biomech 24S:31–39
Herzog W, Leonard TR, Guimaraes ACS (1993) Forces in gastrocnemius, soleus, and plantaris tendons of the freely moving cat. J Biomech 26:945–953
Herzog W, Hasler EM, Maitland ME, Suter E, Leonard TR, Muller C (1998) In vivo mechanics and in situ stability of the anterior cruciate ligament-deficient knee. An animal model of osteoarthritis. Sportverletz Sportschaden 14.2:67–74
Hasler EM, Herzog W (1998) Quantification of in vivo patellofemoral contact forces before and after ACL transection. J Biomech 31:37–44
Herzog W, Hasler EM, Leonard TR (2000) Experimental determination of in vivo pressure distribution in biologic joints. J Musculoskeletal Res 4:1–7
Clark AL, Herzog W, Leonard TR (2002) Contact area distribution in the feline patellofemoral joint under physiologically meaningful loading conditions. J Biomech 35:53–60
Herzog W, Wu JZ, Leonard TR, Suter E, Diet S, Muller C, Mayzus P (1998) Mechanical and functional properties of cat knee articular cartilage 16 weeks post ACL transection. J Biomech 31:1137–1145
Krebs DE, Staples WH, Cuttita D, Zickel RE (1983) Knee joint angle: its relationship to quadriceps femoris activity in normal and postarthrotomy limbs. Arch Phys Med Rehabil 64:441–447
Suter E, Herzog W, Bray RC (1998) Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech 13:314–319
Merton PA (1954) Voluntary strength and fatigue. J Physiol 123:553–564
Belanger AY, McComas AJ (1981) Extent of motor unit activation during effort. J Appl Physiol 51:1131–1135
Suter E, Herzog W, De Souza K, Bray R (1998) Inhibition of the quadriceps muscles in patients with anterior knee pain. J Appl Biomech 14:360–373
Clark A, Herzog W, Matyas JR, Barclay L, Leonard TR (2002) Proceedings of the IV World Congress of Biomechanics, Calgary, AB, Canada. CD ROM
Suter E, Herzog W (2000) Does muscle inhibition after knee injury increase the risk of osteoarthritis. Exerc Sport Sci Rev 28:15–18
Acknowledgements
The research described here has been continuously funded since 1992 by the Canadian Institutes of Health Research (formerly the Medical Research Council of Canada) and the Arthritis Society of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herzog, W., Longino, D. & Clark, A. The role of muscles in joint adaptation and degeneration. Langenbecks Arch Surg 388, 305–315 (2003). https://doi.org/10.1007/s00423-003-0402-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-003-0402-6