Abstract
Purpose
The Clara cell protein CC16, secreted from Clara cells in the lung, is discussed as a potential biomarker for toxic effects on the airways. An increased concentration of CC16 in serum may be caused by increased permeability of the lungs. To investigate the changes in P-CC16 in response to an intense exercise bout performed at different times of day (9 am and 4 pm) of highly trained individuals.
Method
Using a crossover randomized design, 8 runners (mean VO2max 71 ml kg−1 min−1, SD 6) performed a 10-km time trial run, at 9 am and 4 pm, in an environmental chamber set at 6 °C. Lung function tests and blood sampling occurred at baseline, immediately post and 1 h post time trial.
Result
Diurnal differences (P < 0.05) were found for blood neutrophil and lymphocyte counts; with higher values at 4 pm. P-CC16 was higher at the pre- and post-trial time point at 9 am compared to 4 pm. Lung function was not different between or within trials.
Conclusion
Morning trial in cold condition caused more physiological strain compared to the same trial in the evening. However, this extra stress caused by zeitgebers could be a useful strategy for athletes, coaches, and general population to improve their running performance and protect their health in cold conditions in the long-term plan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highly trained athletes can be repeatedly exposed to cold air, inhalant irritants and allergens during training and racing (Helenius et al. 2005). This exposure can result in airway epithelial injury (Bolger et al. 2011), as a result of dehydration and physical stress applied to the airways during maximum effort in extreme environmental conditions (Bolger et al. 2011). Wilber et al. (2000) reported that for the seven sports evaluated on the 1998 USA Winter Olympic Team, 23% of the athletes demonstrated airway inflammation.
Several studies have reported that the risk of upper respiratory tract infection (URTI) is elevated during periods of heavy training and in the period of one to two weeks following participation in endurance races (Nieman et al. 1991; Foster 1998). Moreover, the upper respiratory tract, specifically the nasal cavity, is the first target of defence against ambient cold and irritants. Therefore, the nasal cavity represents an ideal site for the development of biomarkers of events related to airborne exposures. Airway inflammation can be detected by an increase in inflammatory markers, such as neutrophils, macrophages, inflammatory cytokines and Clara cell protein (CC16) (Bonsignore et al. 2001). CC16 is a biomarker of airway respiratory stress (Gomes et al. 2010). This protein, initially described in the epithelium of the tracheobronchial tree as a secretory product from non-ciliated Clara cells, diffuses passively from the respiratory tract into plasma and is excreted via the urinary tract (Bernard et al. 1997).
The functions of Clara cells are mainly oriented to the protection of the respiratory tract, decreasing inflammation of the airways and protecting the respiratory tract against oxidative stress (Gomes et al. 2010). Also, CC16 found in plasma has been shown to be a sensitive marker for the early detection of increases in the permeability of the lung epithelial barrier (Hermans and Bernard 1999). To date, no studies have looked at the relationship between CC16, circadian rhythmicity and sports performance. However, a limited number of studies look at the diurnal variation in CC16 within a healthy population (Helleday et al. 2006; Andersson et al. 2007). Andersson et al.’s (2007) finding was in agreement with Helleday et al. (2006) that showed a decrease in serum CC16 concentration during the daytime.
The novelty of this study is that the participants were high-level runners, tested at two different times of the day in a cold environmental condition. An array of immunological and physiological data analysed offers an important overview on the effect of exercising at a distinct time of day in cold condition. It is relevant for coaches and athletes to be aware of how the time of day can affect various physiological and immunological responses and running performance. This knowledge can allow for improved tailoring of training programs and specific activities to the time of day that generates maximum effectiveness. Furthermore, understanding the diurnal cycle of athlete performance could also be applied to testing sessions and competition times.
Materials and methods
Eight male endurance runners (mean ± SD 32 ± 5 years, 71 ± 6 mlO2 kg−1 min−1, 69 ± 4 kg and 178 ± 5.7 cm) took part in this study. The participants were asked to complete a general medical history questionnaire and a specific medical questionnaire. All participants provided fully informed written consent before engaging with the experiment and were free to withdraw at any stage.
Preliminary measurements
VO2max test was conducted as described by Gomes et al. (2011), using online gas analysis (CPX MedGraphics, Oldham, UK); the VO2max was started at an initial speed of 10 km h−1 with 0% gradient. Every 3 min, the treadmill speed was increased by 3 km h−1 until achieving a maximum speed of 16 km h−1. At this stage and after running 3 min, the treadmill gradient increased by 2.5% every minute until the runner reached maximum fatigue.
Exercise
The exercise protocol consisted of a 10-km time trial run on a treadmill (Woodway, ergo ELG 55, Weil am Rhein, Germany), where the athletes had to complete the distance in the shortest amount of time. Two exercise trials were performed in a randomized order, one at 9 am and the other at 4 pm, with at least a 48-h interval between the trials. The trials were performed in an environmental chamber (Weis-Gallenkamp, UK) where the temperature was controlled at 6 °C with 60% relative humidity (the average British winter temperature 4.4 °C, http://metoffice.gov.uk). During the run, subjective ‘Ratings of Perceived Exertion’ (RPE), on a scale of 6–20 (Borg, 1998), heart rate (Polar Electro, Finland), and running speed were recorded at the end of each km. The athletes had free control of their running speed, without having access to the value of the speed. Also, they were allowed water ad libitum throughout the trial and were advised to refrain from intense physical activity 24 h prior to the exercise trials.
Sample collection
Lung function tests and blood sample collection were conducted pre, post and 1 h post-trial. Lung function tests (FVC, FEV1, FEV1R, PEF, and FEF25-75) were measured using a spirometer (Compact II: Type C, Vitalograph Ltd., UK). The participants performed the test three times and the best values were recorded.
Blood samples were collected in 6-ml vacuum tubes containing EDTA (Becton–Dickinson, Oxford, UK). The samples were collected by venepuncture from a prominent antecubital vein. Cell counts and differentiation were performed with the hematoanalyser (Sysmex, xs 1000i.USA). The samples were then centrifuged for 20 min at 3000g at 4 °C (Mistral 2000R, Sanyo, Leicester, UK) and the plasma was aliquoted into eppendorfs (500 ml) and immediately stored at −80 °C until further analysis.
Biochemical analysis
Plasma CC16 (P-CC16) was measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Abingdon, UK) in accordance with the manufacturer’s instructions. Also, plasma volume changes (dehydration) were calculated using the method as described in Dill and Costill (1974).
Statistical analysis
The method of Altman (1998) was used to determine the number of subjects required for this study. Data were analysed using parametric statistics following confirmation of normal data distribution by Shapiro–Wilks’ tests. The effects of diurnal variation on physiological and biochemical measures were determined using a two-way repeated-measures ANOVA incorporating one within- (state: pre vs. post vs. 1 h post-trial) and one between-subjects (group: 9 am vs. 4 pm) factor. Statistical analyses were conducted using Minitab statistical software version 15 (Minitab Inc., UK); the alpha level was established at P < 0.05 and all values are reported as mean ± SD unless otherwise stated.
Results
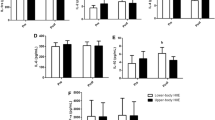
There was no difference in the athlete’s performance time in the two conditions (Table 1). During exercise, HR demonstrated a diurnal variation (Fig. 1b), with significantly lower values (P < 0.05) at 4 pm compared to 9 am: were the mean HR difference was observed from the first km to 9 km. The resting and maximum at 10 km point HR values, however, were not different.
Diurnal variation in P-CC16, WBC, neutrophil and lymphocyte
The results show that neither rest nor zeitgeber has an effect on P-CC16 in participant’s diurnal variation (Fig. 2a). P-CC16 at 9 am shows a significant difference between time point trials (F 2,12 = 3.97, P < 0.001, partial η 2 = 0.4) with Bonferroni-adjusted, post hoc test revealing that the P-CC16 has significantly changed pre to post and post to 1 h post-trial (P = 0.05 and P = 0.05) (Fig. 2a). A significant diurnal difference (P < 0.05) was found in blood neutrophil counts, with higher counts at 4 pm compared to at 9 am at the three measured time intervals (Fig. 2b). Likewise, a significant diurnal difference in blood lymphocyte count was also observed with greater counts at 4 pm compared to 9 am at the three time points (Fig. 2c).
No differences were found between trials or time points (P > 0.05) for the tested lung functions (Table 2).
Diurnal variation in red blood cell variables and platelet
The mean concentration of RBC, HGB, HCT and PLT showed both no diurnal variation and no significant difference between trials times point (Table 3).
Diurnal variation in plasma volume (dehydration)
Plasma volume was 53.8 + 5.32 and 52.2 ± 4.95 ml at 9 am and 4 pm, respectively. An increase of 0.26 g 100 ml−1 in HGB concentration was reported at 9 am and an increase by 0.06 g 100 ml−1 at 4 pm. There was no diurnal variation observed in plasma volume dehydration (P = 0.80); the plasma volume was 3% lower in the evening compared to the morning.
Discussion
This study examined the effect of the time of the day on 10-km treadmill running at 6 °C. This represents the typical mean winter conditions within the UK and Western Europe (http://metoffice.gov.uk). The physiological parameter of HR was more affected by zeitgeber in the morning compared to the evening time-point. However, no statistical differences were found for running performance between these time points. Interestingly, a diurnal variation was observed for neutrophil and lymphocyte counts.
Athletes’ HR was significantly higher in the morning trial, which indicates that participants in the morning showed higher physical demand during this trial compared to the evening. Also, from a clinical perspective, the morning HR may be a cause for concern for cardiovascular patients that are attempting to practise exercise in the morning in cold condition.
RBC total counts, HCT, HGB, PLT, lung function parameters and plasma volume (dehydration) did not show either a diurnal significant difference between morning and evening trials or at any time point during the trials. This result corroborates with Simpson et al. (2007) where RBC, HCT and HGB did not change in fourteen London marathon finishers.
To the best to our knowledge, this is the first study investigating the diurnal difference on P-CC16 in highly trained runners (>70 VO2max). The concentration of P-CC16 was found to not be affected by the time of the day. This is in disagreement with the published findings of Helleday et al. (2006) who reported a decrease in P-CC16 concentration during daytime, with significant drop between 11.30 am and 10 pm. In contrast, Andersson et al. (2007) found an increase in the concentration of CC16 in urine throughout the day, with significant increase 20 h after exposure to wood smoke. Nevertheless, this protein can be used as a specific biomarker of the airway epithelium integrity and an approach to estimate the degree of lung epithelial injury (Gomes et al. 2011), thus identifying respiratory tract inflammation which is the most common medical condition affecting both highly trained and elite athletes, in particular, those participating in endurance events (Bermon 2007). It is known that environmental conditions impact on the degree of airway epithelial disruption during high-level exercise (Bolger et al. 2011 ). When cold air was inhaled, there was a rise in CC16. Moreover, in our study, the degree of leakage of CC16 could be directed related to the cold environment; this corresponds with Bolger et al. (2011) where after 8 min of exercise in dry air causing an epithelial injury in the subjects taking part in the study.
In the present study, we did not aim to assess reasons for the diurnal variation in the P-CC16 concentrations, and nothing is known about the underlying mechanisms here. We can speculate on a few possible explanations for the higher phase response which was more pronounced in the morning trial. According to McAuley and Matthay (2009), a decrease in P-CC16 concentration could reflect the differentiation of distal lung epithelial cells into alveolar epithelial cells as part of the repair processes. Another explanation could be a difference in transepithelial leakage due to cyclic changes in the tightness of the epithelial tight junctions. Addressing in detail the mechanisms behind variation in P-CC16 conventions is an important aspect to consider for future research.
A significant diurnal difference was observed in total white blood cells, neutrophil and lymphocyte counts. In contrast, monocytes, basophil and eosinophil are not affected by circadian rhythm. Monocytes show a significance difference pre- to post at 4 pm; whereas eosinophil and basophil were significant at both times of the day increasing from pre- to post-trial. As expected, the exercise bout was sufficient to promote a change in lymphocyte counts increasing immediately post-trial compared to those pre-trial or at 1 h post-trial at both times of the day. This result supports the findings of other published work: that intense exercise suppresses the immune system (Simpson et al. 2007; Walsh et al. 2011). Similarly, as anticipated, neutrophil counts significantly increased 1 h post-trial compared to pre- and post-trial at both times of the day. This post-trial increase in neutrophils and lymphocytes is considered to result in an “open window” of decreased host protection, which can last between 3 and 72 h and represents a vulnerable time period for the individual contracting and developing an infection (Nieman and Bishop 2006). The higher neutrophil, lymphocyte counts at 4 pm compared to 9 am is possibly due to the athletes’ daily winter exposure and the cold environment. Another possibility may be the influence of the endocrine hormones as seen with regards to other immune cells (Dimitrov et al. 2009). Both chemical and nutritional interventions have been recommended for athletes to minimize potential negative changes in immunity during periods of intensity training.
It is well known that circadian rhythms cause narrow bronchial calibre in humans in the early hours of the morning (4 am); this may lead to high immunological morning suppression. This article has provided evidence to support the hypothesis that strenuous exercise alters the immune system in well-trained athletes; this dysfunction has reported to lead to an URTI (Nieman et al. 1991; Foster 1998). This study found that lung inflammation is more severe in the morning as evidenced by higher CC16. Previous studies have found links between CC16 and epithelium injury, as well as URTI and epithelial injury (Nieman et al. 1991; Foster 1998). This study did not investigate URTI, however, future research should consider the possibility of time of day variation in URTI in line with lung inflammation and epithelial injury that is greater in the mornings. Furthermore, neutrophils phase response was higher at 9 am when epithelial damage was also reported to be associated with mobilisation of neutrophils in the airway (Yoshihara et al. 2006). In contrast, other immunological parameters such as lymphocytes occur in the afternoon, were this variable can be linked also to URTI.
It can be concluded that the morning trial in a cold condition caused more physiological and immunological strain compared to the same trial in the evening. However, this extra stress caused by zeitgebers could be a useful strategy for athletes, coaches, and general population to improve running performance (fitness) in cold condition in the long-term plan.
Perspective
The athletes in this study were highly fit (mean VO2max <71 ml kg−1 min−1), training, mostly, twice-a-day during the pre-race season. However, exercising at high intensity for a prolonged period of time with insufficient recovery can reduce the body’s ability to fight infection at any time of the day. Running performance was not affected despite the time of the day, most likely due to the subjects’ standard (S-shape). Athletes, coaches and event organisers can program their race events or trainings to target the time when athletes perform best. There is a need to look at psychological factors that may affect running performance in elite athletes. Furthermore, addressing in detail the mechanisms behind variation in P-CC16 conventions is an important aspect to consider for future research.
Abbreviations
- CC16:
-
Clara cell 16
- ELISA:
-
Enzyme-linked immunosorbent assay
- FEF:
-
Forced expiratory flow
- FEF25–75 :
-
Forced expiratory flow 25–75%
- FEV1:
-
Forced expiratory volume in 1 s
- FEV1R:
-
Forced expiratory volume in 1 s ratio
- FVC:
-
Forced vital capacity
- HCT:
-
Haematocrit
- HGB:
-
Haemaglobin
- RBC:
-
Red blood cell
- PLT:
-
Platelet
- WBC:
-
White blood cells
References
Altman DG (1998) Confidence intervals for the number needed to treat. Brit J Sport Med 317:1309–1312
Andersson L, Lundberg P-A, Barregard L (2007) Methodological aspects on measurement of Clara cell protein in urine as a biomarker for airway toxicity, compared with serum levels. J Appl Toxicol 27(1):60–66
Bermon S (2007) Airway inflammation and upper respiratory tract infection in athletes: is there a link? Exerc Immunol Rev 13:6–14
Bernard A, Hermans C, VanHoute G (1997) Transient increase of serum Clara cell protein (CC16) after exposure to smoke. Occup Environ Med 54(1):63–65
Bolger C, Tufvesson E, Anderson SD, Devereux G, Ayres JG, Bjermer L, Sue-Chu M, Kippelen P (2011) Effect of inspired air conditions on exercise-induced bronchoconstriction and urinary CC16 levels in athletes. J Appl Physiol 111(4):1059–1065
Bonsignore MR, Morici G, Riccobono L, Insalaco G, Bonanno A, Profita M, Paterno A, Vassalle C, Mirabella A, Vignola AM (2001) Airway inflammation in nonasthmatic amateur runners. Am J Physiol Lung Cell Mol Physiol 281(3):L668–L676
Borg G (1998) Perceived exertion and pain scales. Human Kinetics, Champaign
Dill DB, Costill DL (1974) calculation of percentage changes in volumes of blood, plasma and red cells in hydration. J Appl Physiol 37(2):247–248
Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T (2009) Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 113(21):5134–5143
Foster C (1998) Monitoring training in athletes with reference to overtraining syndrome. Med Sci Sports Exerc 30(7):1164–1168
Gomes CE, Stone V, Florida-James G (2010) The impact of ozone pollution, heat and humidity on the performance and lung function of male athletes during an 8 km time trial run. Eur J Appl Physiol 110:199–205
Gomes CE, Allgrove JE, Florida-James G, Stone V (2011) Effect of vitamin supplementation on lung injury and running performance in a hot, humid, and ozone-polluted environment. Scand J Med Sci Sports 21(6):E452–E460
Helenius I, Lumme A, Haahtela T (2005) Asthma, airway inflammation and treatment in elite athletes. Sports Med 35(7):565–574
Helleday R, Segerstedt B, Forsberg B, Mudway I, Nordberg G, Bernard A, Blomberg A (2006) Exploring the time dependence of serum Clara cell protein as a biomarker of pulmonary injury in humans. Chest 130(3):672–675
Hermans C, Bernard A (1999) Lung epithelium-specific proteins. Characteristics and potential applications as markers (State of art). Am J Respir Crit Care Med 159:646–678
McAuley DF, Matthay MA (2009) Clara cell protein CC16: a new lung epithelial biomarker for acute lung injury. Chest 135(6):1408–1410
Nieman DC, Bishop NC (2006) Nutritional strategies to counter stress to the immune system in athletes, with special reference to football. J Sports Sci 24(7):763–772
Nieman DC, Nehlsencannarella SL, Donohue KM, Chritton DBW, Haddock BL, Stout RW, Lee JW (1991) The effect of acute moderate exercise on leukocyte and lymphocyte subpopulations. Med Sci Sports Exerc 23(5):578–585
Simpson RJ, Florida-James GD, Whyte GP, Middleton N, Shave R, George K, Guy K (2007) The effects of marathon running on expression of the complement regulatory proteins CD55 (DAF) and CD59 (MACIF) on red blood cells. Eur J Appl Physiol 99(2):201–204
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goete L, Rogers CJ, Northoff H, Abbasi A, Simon P (2011) Position statement part one: immune function and exercise. Exerc Immunol Rev 17:6–63
Wilber RL, Rundell KW, Szmedra L, Jenkinson DM, Im J, Drake SD (2000) Incidence of exercise-induced bronchospasm in Olympic winter sport athletes. Med Sci Sports Exerc 32(4):732–737
Yoshihara S, Yamada Y, Abe T, Lindén A, Arisaka O (2006) Association of epithelial damage and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin Exp Immunol 144(2):212–216
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author of this publication has research support and was sponsored by Edinburgh Napier University. The authors were wholly responsible for the research conducted in this paper and present the work without conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Edinburgh Napier University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boukelia, B., Fogarty, M.C., Davison, R.C.R. et al. Diurnal physiological and immunological responses to a 10-km run in highly trained athletes in an environmentally controlled condition of 6 °C. Eur J Appl Physiol 117, 1–6 (2017). https://doi.org/10.1007/s00421-016-3489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3489-5