Abstract
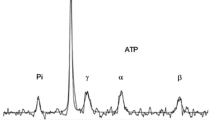
Maximal exercise elicits systemic acidosis where venous pH can drop to 6.74 and here we assessed how much lower the intracellular value (pHi) might be. The wrist flexor muscles are intensively involved in rowing and 31P-magnetic resonance spectroscopy allows for calculation of forearm pHi and energy metabolites at high time resolution. Arm venous blood was collected in seven competitive rowers (4 males; 72 ± 5 kg; mean ± SD) at rest and immediately after a “2,000 m” maximal rowing ergometer effort when hemoglobin O2 saturation decreased from 51 ± 4 to 29 ± 9% and lactate rose from 1.0 ± 0.1 to 16.8 ± 3.6 mM. Venous pH and pHi decreased from 7.43 ± 0.01 to 6.90 ± 0.01 and from 7.05 ± 0.02 to 6.32 ± 0.19 (P < 0.05), respectively, while the ratio of inorganic phosphate to phosphocreatine increased from 0.12 ± 0.03 to 1.50 ± 0.49 (P < 0.05). The implication of the recorded intravascular and intracellular acidosis and the decrease in PCr is that the anaerobic contribution to energy metabolism during maximal rowing corresponds to 4.47 ± 1.8 L O2, a value similar to that defined as the “accumulated oxygen deficit”. In conclusion, during maximal rowing the intracellular acidosis, expressed as proton concentration, surpasses ~4-fold the intravascular acidosis, while the resting gradient is ~2.



Similar content being viewed by others
References
Achten E, Van Cauteren M, Willem R, Luypaert R, Malaisse WJ, Van Bosch G, Delanghe G, De Meirleir K, Osteaux M (1990) 31P-NMR spectroscopy and the metabolic properties of different muscle fibers. J Appl Physiol 68:644–649
Bangsbo J (1998) Quantification of anaerobic energy production during intense exercise. Med Sci Sports Exerc 30:47–52
Bangsbo J, Michalsik L, Petersen A (1993) Accumulated O2 deficit during intense exercise and muscle characteristics of elite athletes. Int J Sports Med 14:207–213
Bergström J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest 12(Suppl 68):11–13
Boushel R, Madsen P, Nielsen HB, Quistorff B, Secher NH (1998a) Contribution of pH, diprotonated phosphate and potassium for the reflex increase in blood pressure during handgrip. Acta Physiol Scand 164:269–275
Boushel R, Pott F, Madsen P, Rådegran G, Nowak M, Quistorff B, Secher N (1998b) Muscle metabolism from near infrared spectroscopy during rhythmic handgrip in humans. Eur J Appl Physiol Occup Physiol 79:41–48
Brøns C, Jensen CB, Storgaard H, Alibegovic A, Jacobsen S, Nilsson E, Astrup A, Quistorff B, Vaag A (2008) Mitochondrial function in skeletal muscle is normal and unrelated to insulin action in young men born with low birth weight. J Clin Endocrinol Metab 93:3885–3892
Brooks GA (2002) Lactate shuttles in nature. Biochem Soc Trans 30:258–264
Cairns SP (2006) Lactic acid and exercise performance: culprit or friend? Sports Med 36:279–291
Costa Leite T, Da Silva D, Guimarães Coelho R, Zancan P, Sola-Penna M (2007) Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem J 408:123–130
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74:49–94
Green S, Dawson B (1993) Measurement of anaerobic capacities in humans. Definitions, limitations and unsolved problems. Sports Med 5:312–327
Hermansen L, Osnes JB (1972) Blood and muscle pH after maximal exercise in man. J Appl Physiol 32:304–308
Hoch JC, Stern SS (1996) NMR data processing. Wiley, Hoboken
Juel C (2008) Regulation of pH in human skeletal muscle: adaptations to physical activity. Acta Physiol 193:17–24
Krogh A, Lindhard J (1920) The changes in respiration at the transition from work to rest. J Physiol 53:431–437
Lauritsen TL, Grunnet N, Rasmussen A, Secher NH, Quistorff B (2002) The effect of hepatectomy on glucose homeostasis in pig and in man. J Hepatol 36:99–104
Lundsgaard E (1932) Betydningen af faenomenet maelkesyrefrie muskelkontraktoner for opfattelsen af muskelkontraktioners kemi. Danske Hospitalstidende 75:84–95
Margaria R, Edwards HT, Dill DB (1933) The possible mechanisms of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am J Physiol 106:689–715
Medbø JI, Mohn AC, Tabata I, Bahr R, Vaage O, Sejersted OM (1988) Anaerobic capacity determined by maximal accumulated O2 deficit. J Appl Physiol 64:50–60
Mizuno M, Secher NH, Quistorff B (1994) 31P-NMR spectroscopy, rsEMG, and histochemical fiber types of human wrist flexor muscles. J Appl Physiol 76:531–538
Nielsen HB (1999) pH after competitive rowing: the lower physiological range? Acta Physiol Scand 165:113–114
Nielsen OB, Paoli F, Overgaard K (2001) Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 536:161–166
Nielsen HB, Hein L, Svendsen LB, Secher NH, Quistorff B (2002) Bicarbonate attenuates intracellular acidosis. Acta Anaesthesiol Scand 46:579–584
Nielsen HB, Febbraio MA, Ott P, Krustrup P, Secher NH (2007) Hepatic lactate uptake versus leg lactate output during exercise in humans. J Appl Physiol 103:1227–1233
Pedersen TH, Nielsen OB, Lamb GD, Stephenson GD (2004) Intracellular acidosis enhances the excitability of working muscle. Science 305:1144–1147
Pripstein LP, Rhodes EC, McKenzie DC, Coutts KD (1999) Aerobic and anaerobic energy during a 2-km race simulation in female rowers. Eur J Appl Physiol 79:491–494
Quistorff B, Secher NH, Van Lieshout JJ (2008) Lactate fuels the human brain during exercise. FASEB J 22:3443–3449
Rasmussen UF, Rasmussen HN, Krustrup P, Quistorff B, Saltin B, Bangsbo J (2001) Aerobic metabolism of human quadriceps muscle: in vivo data parallel measurements on isolated mitochondria. Am J Physiol Endocrinol Metab 280:E301–E330
Ratkevicius A, Quistorff B (2002) Metabolic costs of force generation for constant-frequency and catchlike-inducing electrical stimulation in human tibialis anterior muscle. Muscle Nerve 25:419–426
Renaud JM (1989) The effect of lactate on intracellular pH and force recovery of fatigued sartorius muscles of the frog, Rana pipiens. J Physiol 416:31–47
Sacks J, Sacks WC (1937) Blood and muscle lactic acid in the steady state. Am J Physiol 118:697–702
Sahlin K, Alvestrand A, Brandt R, Hultman E (1978) Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol 45:474–480
Secher NH, Volianitis S (2006) Are the arms and legs in competition for cardiac output? Med Sci Sports Exerc 38:1797–1803
Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J (1977) Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand 100:288–297
Secher NH, Petersen S, Grimby G (1984) Effect of tubocurarine on static and dynamic muscle contractions in man. Acta Physiol Scand 120:251–255
Secher NH, Rube N, Elers J (1988) Strength of two- and one-leg extension in man. Acta Physiol Scand 134:333–339
Volianitis S, Secher NH (2009) Rowing, the ultimate challenge to the human body—implications for physiological variables. Clin Physiol Funct Imaging 29:241–244
Westerblad H, Allen DG (1992) Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 449:49–71
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Volianitis, S., Secher, N.H. & Quistorff, B. The intracellular to extracellular proton gradient following maximal whole body exercise and its implication for anaerobic energy production. Eur J Appl Physiol 109, 1171–1177 (2010). https://doi.org/10.1007/s00421-010-1451-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1451-5