Abstract
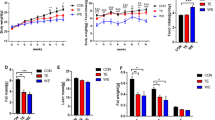
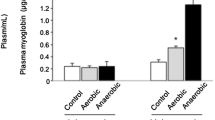
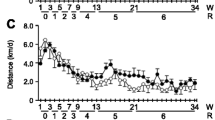
The aims of the present study were as follows: (1) to examine the adaptational changes to chronic endurance voluntary exercise and (2) to investigate the effects of amino acid supplementation on the adaptational changes induced by endurance training in hindlimb (gastrocnemius, tibialis, soleus) and respiratory (diaphragm) muscles of mice. Male C57Bl6 mice were divided in four groups: control sedentary, sedentary supplemented with amino acid mixture (BigOne, 1.5 mg g day−1 in drinking water for 8 weeks), running (free access to running wheels for 8 weeks), and running supplemented with amino acid mixture. Myosin heavy chain (MHC) isoform distribution was determined in all muscles considered. Fiber cross-sectional area (CSA) was measured in the soleus muscle. In all muscles except the tibialis, endurance training was associated with an overall shift towards the expression of slower MHC isoforms. Amino acid supplementation produced a shift towards the expression of faster MHC isoforms in the soleus and diaphragm muscles, and partially antagonized the effects of training. Immunohistochemical analysis of CSA of individual muscle fibers from the soleus muscle suggests that voluntary running produced a decrease in the size of type 1 fibers, and amino acid supplementation during training resulted in an increase in size in both type 1 and type 2A fibers. Collectively, these results suggest that the endurance adaptations induced by voluntary running depend on the muscle type, and that amino acid supplementation is able to modulate both fiber size and MHC isoform composition of skeletal muscles in sedentary and exercised mice.



Similar content being viewed by others
References
Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA (2001) Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90:1900–1908
Antonio J, Sanders MS, Ehler LA, Uelmen J, Raether JB, Stout JR (2000) Effects of exercise training and amino-acid supplementation on body composition and physical performance in untrained women. Nutrition 16:1043–1046
Avraham Y, Bonne O, Berry EM (1996) Behavioral and neurochemical alterations caused by diet restriction—the effect of tyrosine administration in mice. Brain Res 732:133–144
Avraham Y, Hao S, Mendelson S, Berry EM (2001) Tyrosine improves appetite, cognition, and exercise tolerance in activity anorexia. Med Sci Sports Exerc 33:2104–2110
Baldwin KM, Haddad F (2001) Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol 90:345–357
Baldwin KM, Cooke DA, Cheadle WG (1977) Time course adaptations in cardiac and skeletal muscle to different running programs. J Appl Physiol 42:267–272
Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ (1989) Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1–13C]leucine. Clin Sci (Lond) 76:447–454
Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR (1995) Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 268:E514–E520
Biolo G, Tipton KD, Klein, S Wolfe RR (1997) An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 273:E122–E129
Blomstrand E, Saltin B (2001) BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am J Physiol 281: E365–E374
Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA (1989) Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand 136:473–481
Blomstrand E, Hassmen P, Ekblom B, Newsholme EA (1991) Administration of branched-chain amino acids during sustained exercise—effects on performance and on plasma concentration of some amino acids. Eur J Appl Physiol 63:83–88
Blomstrand E, Andersson S, Hassmen P, Ekblom B, Newsholme EA (1995) Effect of branched-chain amino acid and carbohydrate supplementation on the exercise-induced change in plasma and muscle concentration of amino acids in human subjects. Acta Physiol Scand 153:87–96
Booth FW, Thomason DB (1991) Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev 71:541–585
Bottinelli R, Reggiani C (2000) Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73:195–262
Bottinelli R, Schiaffino S, Reggiani C (1991) Force–velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol (Lond) 437:655–672
Calders P, Matthys D, Derave W, Pannier JL (1999) Effect of branched-chain amino acids (BCAA), glucose, and glucose plus BCAA on endurance performance in rats. Med Sci Sports Exerc 31:583–587
Carli G, Bonifazi M, Lodi L, Lupo C, Martelli G, Viti A (1992) Changes in the exercise-induced hormone response to branched chain amino acid administration. Eur J Appl Physiol 64:272–277
Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA (1987) Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest 80:1784–1793
D’Antona G, Pellegrino MA, Polla B, Ricci S, Conti F, Bottinelli R (2001) Effects of voluntary wheel running on skeletal muscle of mice with standard diet or with amino acid supplementation. J Muscle Res Cell Motil 22:567
Darveau CA, Suarez RK, Andrews RD, Hochachka PW (2002) Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417:166–170
Deijen JB, Orlebeke JF (1994) Effect of tyrosine on cognitive function and blood pressure under stress. Brain Res Bull 33:319–323
Deijen JB, Wientjes CJ, Vullinghs HF, Cloin PA, Langefeld JJ (1999) Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res Bull 48:203–209
Demirel HA, Powers SK, Naito H, Hughes M, Coombes JS (1999) Exercise-induced alterations in skeletal muscle myosin heavy chain phenotype: dose–response relationship. J Appl Physiol 86:1002–1008
Dohm MR, Richardson CS, Garland TJr (1994) Exercise physiology of wild and random-bred laboratory house mice and their reciprocal hybrids. Am J Physiol 267:R1098–R1108
Dudley GA, Abraham WM, Terjung RL (1982) Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol 53:844–850
Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M (2001) Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol (Lond) 535:301–311
Fitts RH, Widrick JJ (1996) Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24:427–473
Fitts RH, Costill DL, Gardetto PR (1989) Effect of swim exercise training on human muscle fiber function. J Appl Physiol 66:465–475
Fitts RH, McDonald KS, Schluter JM (1991) The determinants of skeletal muscle force and power: their adaptability with changes in activity pattern. J Biomech 24:111–122
Fitzsimons DP, Diffee GM, Herrick RE, Baldwin KM (1990) Effects of endurance exercise on isomyosin patterns in fast- and slow-twitch skeletal muscles. J Appl Physiol 68:1950–1955
Garland, TJr, Gleeson TT, Aronovitz BA, Richardson, CS Dohm MR (1995) Maximal sprint speeds and muscle fiber composition of wild and laboratory house mice. Physiol Behav 58:869–876
Georgiades E, Behan WM, Kilduff LP, Hadjicharalambous M, Mackie EE, Wilson J, Ward SA, Pitsiladis YP (2003) Chronic fatigue syndrome: new evidence for a central fatigue disorder. Clin Sci (Lond) 105:213–218
Gibala MJ (2000) Nutritional supplementation and resistance exercise: what is the evidence for enhanced skeletal muscle hypertrophy? Can J Appl Physiol 25:524–535
Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA (2002) Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol 92:313–322
Hickson RC, Galassi TM, Dougherty KA (1983) Repeated development and regression of exercise-induced cardiac hypertrophy in rats. J Appl Physiol 54:794–797
Holloszy JO (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242:2278–2282
Houle-Leroy P, Garland TJr, Swallow JG, Guderley H (2000) Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol 89:1608–1616
Hutton JC, Sener A, Malaisse WJ (1980) Interaction of branched chain amino acids and keto acids upon pancreatic islet metabolism and insulin secretion. J Biol Chem 255:7340–7346
Ishihara A, Inoue N, Katsuta S (1991) The relationship of voluntary running to fibre type composition, fibre area and capillary supply in rat soleus and plantaris muscles. Eur J Appl Physiol 62:211–215
Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O (2002) Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol 93:1301–1309
Kleiber M (1932) Body size and metabolism. Hilgardia 6:315–353
Kriketos AD, Pan DA, Sutton JR, Hoh JF, Baur LA, Cooney GJ, Jenkins AB, Storlien LH (1995) Relationships between muscle membrane lipids, fiber type, and enzyme activities in sedentary and exercised rats. Am J Physiol 269:R1154–R1162
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Levenhagen DK, Carr C, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ (2002) Postexercise protein intake enhances whole-body and leg protein accretion in humans. Med Sci Sports Exerc 34:828–837
Nair KS, Schwartz RG, Welle S (1992) Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol 263:E928–E934
Pansarasa O, D’Antona G, Gualea MR, Marzani B, Pellegrino MA, Marzatico F (2002) “Oxidative stress”: effects of mild endurance training and testosterone treatment on rat gastrocnemius muscle. Eur J Appl Physiol 87:550–555
Pellegrino MA, Canepari M, Rossi R, D’Antona G, Reggiani C, Bottinelli R (2003) Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J Physiol (Lond) 546:677–689
Pette D (1998) Training effects on the contractile apparatus. Acta Physiol Scand 162:367–376
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR (2000) An oral essential amino acid–carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88:386–392
Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE (1989) Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol 66:1250–1257
Schiaffino S, Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76:371–423
Schluter JM, Fitts RH (1994) Shortening velocity and ATPase activity of rat skeletal muscle fibers: effects of endurance exercise training. Am J Physiol 266:C1699–C1673
Smith K, Reynolds N, Downie S, Patel A, Rennie MJ (1998) Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol 275:E73–E78
Sugiura T, Morimoto A, Sakata Y, Watanabe T, Murakami N (1990) Myosin heavy chain isoform changes in rat diaphragm are induced by endurance training. Jpn J Physiol 40:759–763
Sugiura T, Morimoto A, Murakami N (1992) Effects of endurance training on myosin heavy-chain isoforms and enzyme activity in the rat diaphragm. Pflugers Arch 421:77–81
Sullivan VK, Powers SK, Criswell DS, Tumer N, Larochelle JS, Lowenthal D (1995) Myosin heavy chain composition in young and old rat skeletal muscle: effects of endurance exercise. J Appl Physiol 78:2115–2120
Swallow JG, Garland T Jr, Carter PA, Zhan WZ, Sieck GC (1998) Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). J Appl Physiol 84:69–76
Talmadge RJ, Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75:2337–2340
Tibbits G, Koziol BJ, Roberts NK, Baldwin KM, Barnard RJ (1978) Adaptation of the rat myocardium to endurance training. J Appl Physiol 44:85–89
Tipton KD, Ferrando AA, Phillips SM, Doyle DJr, Wolfe RR (1999) Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 276:E628–E634
Van Hall G, Raaymakers JS, Saris WH, Wagenmakers AJ (1995) Ingestion of branched-chain amino acids and tryptophan during sustained exercise in man: failure to affect performance. J Physiol (Lond) 486:789–794
Verger P, Aymard P, Cynobert L, Anton G, Luigi R (1994) Effects of administration of branched-chain amino acids vs. glucose during acute exercise in the rat. Physiol Behav 55:523–526
Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR (1998) Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101:2000–2007
Vukovich MD, Sharp RL, Kesl LD, Schaulis DL, King DS (1997) Effects of a low-dose amino acid supplement on adaptations to cycling training in untrained individuals. Int J Sport Nutr 7:298–309
Wernig A, Irintchev A, Weisshaupt P (1990) Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol (Lond) 428:639–652
Widrick JJ, Trappe SW, Blaser CA, Costill DL, Fitts RH (1996) Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am J Physiol 271:C666–C675
Zhan WZ, Swallow JG, Garland T Jr, Proctor DN, Carter PA, Sieck GC (1999) Effects of genetic selection and voluntary activity on the medial gastrocnemius muscle in house mice. J Appl Physiol 87:2326–2333
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pellegrino, M.A., Brocca, L., Dioguardi, F.S. et al. Effects of voluntary wheel running and amino acid supplementation on skeletal muscle of mice. Eur J Appl Physiol 93, 655–664 (2005). https://doi.org/10.1007/s00421-004-1237-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1237-8