Abstract
Recent evidence indicates that targeting IL-6 provides broad therapeutic approaches to several diseases. In patients with cancer, autoimmune diseases, severe respiratory infections [e.g. coronavirus disease 2019 (COVID-19)] and wound healing, IL-6 plays a critical role in modulating the systemic and local microenvironment. Elevated serum levels of IL-6 interfere with the systemic immune response and are associated with disease progression and prognosis. As already noted, monoclonal antibodies blocking either IL-6 or binding of IL-6 to receptors have been used/tested successfully in the treatment of rheumatoid arthritis, many cancer types, and COVID-19. Therefore, in the present review, we compare the impact of IL-6 and anti-IL-6 therapy to demonstrate common (pathological) features of the studied diseases such as formation of granulation tissue with the presence of myofibroblasts and deposition of new extracellular matrix. We also discuss abnormal activation of other wound-healing-related pathways that have been implicated in autoimmune disorders, cancer or COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Tumours are wounds that do not heal” (Dvorak 1986). Based on gross morphological resemblance, this classical parallel was proposed by Harold F. Dvorak more than three decades ago (in 1986). Notably, the tumour stroma of the vast majority of solid cancers is very similar to the granulation tissue that is indispensable for physiological wound healing. Differences are sometimes very subtle and given by the context. Indeed, since recently, we can follow a multitude of identical molecular mechanisms underlying these pathological conditions and thus supporting this historical parallel more robustly. Therefore, a broader view seems advantageous in distinguishing what makes one mechanism beneficial in healing, while the identical mechanism may represent an advantage for cancer progression and a critical obstacle in the treatment of malignancies.
The COVID-19 pandemic in recent years brought many new challenging inputs to the clinics and biomedical sciences, including pathology. Respiratory complications in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are associated with substantial morbidity and mortality. Interestingly, histopathological examination of pulmonary injury in patients who died from COVID-19 revealed striking morphological similarities to findings in autoimmune diseases, cancer and wound healing (Giacomelli et al. 2021). Hence, in the present review, we define the overlapping histological reaction patterns observed in this clinically heterogeneous and previously not compared group of diseases, emphasizing interleukin (IL)-6 signalling.
From a functional point of view, autoimmunity, cancer, COVID-19 and wound healing share several common features represented by deregulated inflammatory/immune responses. Inflammatory cytokine profiles of patients suffering from severe and critical forms of COVID-19 or various types of cancer revealed a potential crossing point, namely significantly elevated serum levels of pro-inflammatory cytokine IL-6 (Brábek et al. 2020). Accordingly, we proposed IL-6 inhibition as a potential therapeutic strategy for COVID-19-related cytokine release syndrome (Smetana and Brábek 2020; Smetana et al. 2020b). This idea was supported by pre-existing permission to use the inhibitor in human medicine [approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of other conditions]. Indeed, this approach was later adopted to treat severe forms of COVID-19 (Lamontagne et al. 2020). However, clinical results achieved with IL-6 signalling inhibitors are somewhat conflicting in critically ill patients with COVID-19 (Declercq et al. 2021; Majidpoor and Mortezaee 2022). At this point, we can hypothesize that a more stringent definition of inclusion/exclusion criteria of patient enrolment would be beneficial to evaluate the outcome of therapy more objectively. On the other hand, optimal drug timing during combination therapies, which is still not well known and seems to be somewhat underrated, may improve the therapeutic outcomes. In this context, it seems likely that anti-IL-6 therapy can offer certain benefits in at least some patients with COVID-19 if properly indicated.
Skin injury – an example of a simple healing process
Undamaged skin represents a protective barrier against a potentially harmful external environment. The skin barrier restricts the invasion of pathogenic microorganisms (bacteria, fungi, viruses) and prevents the loss of water, crystalloids and proteins. Therefore, acute and chronic wounds pose serious healthcare issues.
Skin injury is an excellent model to study the general aspects of wound healing. When the skin integrity barrier is broken, the barrier function is compromised. The healing process requires an effective interplay of various cells regulated by the timely production of molecular signals to replace the missing tissue. More specifically, the skin is a valuable model for studying the complex epithelial–mesenchymal interactions between the epidermis and dermis. The precisely regulated interplay between regeneration of the epidermis and fibroplastic response of the dermis is a prerequisite for successful healing. Deeper insights into the aspects of healing mechanisms may facilitate the identification of bioinspired therapeutic options (Pollini and Paladini 2020).
Of note, the interfollicular epidermis can regenerate fully in humans. However, dermal architecture repair is not so easy in humans, which leads to scar production. Dermis repair is divided into four predictable phases: blood clotting/haemostasis (which may be part of inflammation), inflammation, proliferation and maturation/remodelling. Once this sequence is completed, re-epithelization finally reconstitutes the barrier integrity (Pastar et al. 2014). Although wound healing is complex and tightly orchestrated, it is also susceptible to interruption and failure, resulting in non-healing/chronic wounds or pathological scars (Čoma et al. 2021).
Traumatic tissue injury is usually associated with haemorrhage resulting in the formation of a stable haemostatic clot of polymerized fibrin, preventing major blood loss by a process called haemostasis. The coagulum temporarily fills the defect (forming a crust) and provisionally protects deeper tissue. The formation of this temporary barrier is initiated by the intrinsic (platelets) and extrinsic coagulation (clotting factors) cascade (Reinke and Sorg 2012).
Following haemostasis, dilatation and permeabilization of vessels allow leucocytes to reach the injury site; thus, the inflammatory phase begins. The main goal of the invading leucocytes is to eliminate pathogens, resolve the inflammation and remove necrotic cells/tissue (Ellis et al. 2018). Immune cells are responsible for removing the tissue debris and preventing wound colonization by microorganisms. These immune cells mainly include granulocytes (neutrophils and eosinophils), macrophages, mast cells, natural killer (NK) cells, B lymphocytes and subsets of T lymphocytes.
Neutrophils, the first and most abundant immune cells infiltrating the wound, play an essential role in debris and pathogen removal (de Oliveira et al. 2016). Monocytes/macrophages are of high importance in wound healing as they play a critical role in resolving acute inflammation. Depending on their polarization, macrophages can enhance the immune response (M1 polarization) or attenuate inflammation (M2 polarization) and coordinate tissue repair. The functional and morphologic outcome of wound healing is highly dependent on the success of inflammation resolution (Wilgus 2020). Persisting inflammation may lead to dysregulation of keratinocyte and fibroblast differentiation and often results in excessive scarring and, in the worst case, even in the formation of a hypertrophic/keloid scar (Landén et al. 2016).
To interact across the wound site and orchestrate the inflammatory activity, immune cells release a broad panel of bioactive factors. These include, but are not limited to, transforming growth factor (TGF)-β, tumour necrosis factor (TNF)-α, interferon (IFN)-γ, vascular endothelial growth factor A (VEGFA), basic fibroblast growth factor (bFGF), a panel of interleukins (IL-4-6, IL-9-13, IL-17, IL-23), chemokines [chemokine C–X–C ligand motif (CXCL)-8, CXCL-12] and numerous proteases (Čoma et al. 2021).
Of note, some of these factors are not produced in the microenvironment specifically by a single cell type. To exemplify this pleiotropy, IL-6 is produced by neutrophils, macrophages, mast cells and T lymphocytes. Similarly, immune cells also widely produce members of the TGF-β family. These factors influence different immune cells in the wound microenvironment simultaneously. Moreover, these signals inevitably also affect local fibroblasts, epithelial cells, and endothelium of capillaries.
Moreover, these cytokines can also leak into the systemic circulation. Measurements of IL-6 serum levels were used to monitor large wound healing (Avazi et al. 2019; Zhang et al. 2020a). Hyperactivation of the immune system was rarely observed in large, infected wounds or extensive burn injury, leading anecdotally to the so-called cytokine storm syndrome (Adamik et al. 1997; Mulder et al. 2021).
Successful resolution of the inflammation enables healing to enter the proliferation phase approximately 2–4 days post-injury (Wokalek and Ruh 1991). The phase includes formation of the so-called granulation tissue. This provisional tissue is composed of persisting recruited inflammatory/immune cells, sprouting endothelium of capillaries and fibroblasts producing extracellular matrix (ECM). Fibroblasts first produce a loose ECM that provides a temporary provisional scaffold for other migrating and proliferating cells (Tracy et al. 2016).
Surprisingly, dermal fibroblasts are a highly heterogeneous population of mesenchymal cells. From a histological point of view, two principal subgroups of fibroblasts, papillary and reticular, have been identified. These two pools differ in their proliferative capacity, ability to contract wounds, and expression profile. Single-cell sequencing further distinguished up to six functionally different subsets of dermal fibroblasts. However, only the population expressing dipeptidyl-peptidase IV (also known as CD26) was responsible for ECM production, and thus could play a crucial role in new tissue formation during wound healing (Vorstandlechner et al. 2020). As expected, the resulting scar is highly dependent on the fibroblast subset present in the wounded site (Griffin et al. 2020).
Of note, such remarkable fibroblast diversity can also result from the developmental origin (Thulabandu et al. 2018). In the craniofacial region, dermal fibroblasts have potentially a dual embryonic origin of cranial neural crest (facial fibroblasts) and cephalic mesoderm. Fibroblasts present in the dorsal skin originate from the somites. Finally, fibroblasts in the ventral flank and limb dermis originate from the lateral plate mesoderm (Wong et al. 2006).
Intriguingly, fibroblasts or fibroblast-like cells can also arise from various cell populations. This includes origin from epithelial cells by epithelial-to-mesenchymal transition (EMT) (Thiery et al. 2009), epidermal stem cells (Li et al. 2016), bone marrow-derived mesenchymal stem cells (Saikia et al. 2020) and circulating fibrocytes (Quan et al. 2006) as well as from endothelial cells by endothelial-to-mesenchymal transition (EndMT) (Piera-Velazquez et al. 2011).
For the wound contraction, it is important that fibroblasts can differentiate into myofibroblasts (Hinz 2016) containing stress fibres allowing them to generate contractile forces facilitating wound closure (Li and Wang 2011; Chitturi et al. 2015). Several cytokines, growth factors, and growth and adhesion-regulating galectins were identified as fibroblast and myofibroblast stimulators. These include TGF-β1, CTGF, FGF, PDGF, IGF and galectin-1 (Bonner et al. 1990; Clark et al. 1997; Grazul-Bilska et al. 2002; Dvořánková et al. 2011; Hung et al. 2013; Lin et al. 2015). Of the cytokines listed above, TGF-β1 has been repeatedly reported as a critical factor for fibroblast differentiation into myofibroblasts (Evans et al. 2003; Zhang et al. 2020b; Macarak et al. 2021) (Fig. 1).
Active scar contains numerous α-smooth muscle actin (SMA)-positive myofibroblasts surrounded by extracellular matrix (a). Myofibroblasts and infiltrating immune cells produce IL-6 (b) and TGF-β1 (c); specificity of the reaction was verified by negative (isotype) control (d). While numerous SMA-positive fibroblasts can be isolated from an early active scar (e), the fibroblasts prepared from a quiescent scar are devoid of SMA expression (f). Nuclei are counterstained with DAPI (scale bar, 100 µm)
In parallel, a process called angiogenesis is in progress. This leads to the formation of new blood vessels, providing nutrients and oxygen to satisfy the demand of rapidly proliferating cells (Eelen et al. 2020). VEGF is the most studied and well-known pro-angiogenic molecule. VEGF was identified as vascular endothelial cell mitogen and regulator of endothelial integrin expression during vessel sprouting. Notably, VEGF may act as a chemokine for macrophages, directly activating them via the VEGF receptor (VEGFR) (Barleon et al. 1996). Therefore, VEGF may be considered as an indirect pro-inflammatory cytokine that promotes excessive scar formation.
The cell interactions occurring during the proliferation phase, namely epithelial–mesenchymal cross-talk between fibroblasts, endothelial cells and keratinocytes, have a major impact on the consequent maturation/remodelling phase of wound healing. Any failure of these interactions can lead to a sub-optimal healing result. The resulting scar may be clinically almost unnoticeable in neonates; however, it is usually more apparent in adults (Borsky et al. 2012). Although this unequal healing capacity observed across different ages is a clinically well-documented phenomenon, its explanation is not simple.
The formation of granulation tissue gradually stops through cell apoptosis and converts the wound to avascular and acellular scar. Untimely, cessation of this process leads to excessive accumulation of fibrotic tissue and formation of, e.g., hypertrophic/keloid scarring. However, keloid formation also requires certain genetic susceptibility (Nyika et al. 2022).
Therefore, optimal healing is based on a dynamic equilibrium of ECM production and degradation by proteolytic enzymes (Čoma et al. 2021). It was proposed that the neonatal fibroblasts maintain some properties similar to mesenchymal stem cells (Mateu et al. 2016), also accompanied by deregulation of TGF-β signalling with low expression of the TGF-β II receptor (Živicová et al. 2017).
Peripheral nerve injury – an example of wound healing in highly specialized tissue
As introduced above, human skin has a relatively simple structure and rapid turnover. It differs from the highly specialized structure of nerves with slow turnover. It is well known that the nervous system lacks some extensive capacity for neural regeneration in humans. Therefore, even peripheral nerve repair and regeneration remain among the greatest challenges in regenerative medicine. However, the staging of skin wound healing can be efficiently applied to the repair and/or regeneration of other body tissues, including peripheral nerves. Indeed, the mechanisms are conserved and surprisingly similar.
For example, the proximal and distal stumps of disconnected peripheral nerve exhibit structural changes analogical to the proliferation phase observed during skin wound healing. They are associated with granular disintegration of axons, including their myelin sheaths distal to the injury. Further, there is an accumulation of fibroblasts extensively producing collagen and appearance of myofibroblasts in the proximal stump. Neural scar formation is a part of the injured nerve regeneration, but the excessive expansion of intra-neural scar tissue often hampers nerve regeneration (Wang et al. 2019; Fertala et al. 2020). Of note, it was recently demonstrated that myofibroblasts play a critical role in the nerve outgrowth/regeneration into the gap of an end-to-end nerve suture (Katenkamp and Stiller 1978; Fertala et al. 2020). On the other hand, numerous myofibroblasts occur in the posttraumatic neuromas of the proximal stump of permanently interrupted nerves, induced by chronic inflammation (Oliveira et al. 2018), and there is a clinically evidenced association with extreme pain due to direct malignant infiltration of nerves (Yan et al. 2012) observed in patients with cancer (Urch and Dickenson 2008; Yeh et al. 2016).
A rapidly growing body of evidence suggests that the immune system and its products play a critical role in the regeneration of the peripheral nervous system (Lindborg et al. 2018; Zigmond and Echevarria 2019; Dubový et al. 2021). The role of immune cell recruitment was well exemplified by the involvement of macrophages in the regeneration of neurons (Niemi et al. 2016) and peripheral nerves (Wofford et al. 2022). In detail, the presence of pro-inflammatory cytokines during the first phase of Wallerian degeneration promotes recruitment of macrophages. Later, these macrophages turn into the M2 phenotype, resolving the inflammation via production of anti-inflammatory cytokines. Therefore, macrophage infiltration into an injured nerve is an essential step favouring regeneration. Indeed, transplantation of M2 macrophages attenuated neuropathy-induced mechanical hypersensitivity (Pannell et al. 2016).
Additionally, immune mediators’ functions can also be demonstrated by the contribution of IL-6 and activation of STAT-3 signalling in the regeneration of primary sensory neurons (Dubový et al. 2019) (Fig. 2). Further, IL-6 also promotes regeneration of injured peripheral nerves (Hirota et al. 1996; Cámara-Lemarroy et al. 2010). However, it can also have deleterious effects if overproduced, as shown in sciatic nerve regeneration (Ma et al. 2019). Under these circumstances, it can also be accompanied by neuropathic pain (Zhou et al. 2016). Mechanistically, the intensity of hyperalgesia correlates here with the increased production of inflammatory mediators, including IL-6 (Patil and Testarelli 2021). This production can be reduced, for example, by cannabinoids and/or flavonoids, with a therapeutically relevant effect, i.e. reduced pain intensity in patients with hyperalgesia (Henshaw et al. 2021; Rao et al. 2021).
Longitudinal cryostat sections through rat sciatic nerve distal to compression for 14 days (a–d). The sections were immunostained with rabbit polyclonal anti-IL-6 (a), anti-IL-6R (b), anti-gp130 (c) or anti-STAT3 (d) (all red signals). Arrowheads indicate the position of activated Schwann cells that displayed immunopositive staining. Representative sections through the fourth lumbar dorsal root ganglion were removed from intact rats (e, g, i, k) and those operated on triplet ligature to compress the sciatic nerve for 14 days (f, h, j, l). The sections were immunostained with rabbit polyclonal antibodies against IL-6 (e, f), IL-6R (g, h), gp130 (i, j) or STAT3 (k, l). Cell nuclei were stained using Hoechst [scale bars, 35 µm (a–d) and 30 µm (e–l), respectively]
Incomplete or incorrect peripheral regeneration of sensory fibres is also associated with neuropathic pain induction (Cobianchi et al. 2014; Xie et al. 2017). From this point of view, immunomodulation (following nerve injury) to improve peripheral nerve regeneration efficiency remains one of the greatest challenges in regenerative medicine. It also has therapeutic potential due to its ability to relieve pain (Li et al. 2022).
Autoimmunity – an injury due to auto-aggression
Autoimmune disease occurs when a specific adaptive immune response targets self-antigens, resulting in chronic inflammation-mediated tissue injury. Paul Ehrlich, a great German immunologist, famously coined the term horror autotoxicus for immune-mediated self-destruction (Ehrlich 1900). The immune system uses a plethora of mechanisms to damage tissues and organs in autoimmune diseases. However, the resulting tissue damage associated with fibrosis and impaired function is a surprisingly frequent feature of these deleterious scenarios.
Scleroderma
Scleroderma is a well-known, potentially lethal devastating autoimmune disease of still not completely understood pathogenesis. It may also be called systemic sclerosis (SSc) because it is characterized by fibrotic changes not only in the skin, but also within visceral organs (van Caam et al. 2018). A substantial increase in the mortality rate of patients with SSc is related to cardiac disease, pulmonary fibrosis and pulmonary hypertension. As this maladaptive immune response develops, it is usually impossible for the immune effector cells to entirely eliminate the antigen. Currently used immunosuppressive treatments are only partially effective in the prevention of disease progression. This treatment is inefficient in the removal of already present fibrous tissue.
Histological examination of skin biopsies of patients with SSc revealed changes in the collagen fibre bundles and increased presence of SMA-positive cells in the dermis. The fibrosis in scleroderma is ultimately driven by myofibroblasts (van Praet et al. 2011). These myofibroblasts are particularly resistant to apoptosis. Their number in the dermis positively correlates with abundant collagen overproduction, enhanced ECM stiffness and up-regulated pro-fibrotic cytokines, resulting in elevated skin hardness and disease progression (Ferreli et al. 2017). In the background of tissue inflammation, myofibroblasts are autonomously activated with TGF-β as the critical factor responsible for SMAD-dependent signalling (Ramirez et al. 2006). However, it has also been noted that the TGF-β signalling loop is insufficient to explain the persisting myofibroblast phenotype (Leask 2013).
IL-6 acts as a pro-fibrotic factor in scleroderma (Pedroza et al. 2011). It is increased in patients with scleroderma and significantly correlates with the disease severity scoring system (Shima et al. 2010). Substantial evidence has been gathered to support the role of IL-6 in the disease activity and the development of cardiopulmonary manifestations in patients with SSc (Distler et al. 2020). In this context, serum IL-6 levels have appeared to be predictive of early disease progression in patients with interstitial lung disease.
It was suggested recently that TGF-β and IL-6 pathways could cooperate in fibrous disorders, e.g. idiopathic pulmonary fibrosis. There is a growing body of evidence that lung fibroblasts express high baseline levels of both canonical and IL-6 trans-signalling components, leading to indirect TGF-β pathway activation and disease progression (Epstein Shochet et al. 2020). Therefore, IL-6 inhibitors may prevent early lung disease (Khanna et al. 2020). Although tocilizumab in a phase III clinical trial did not achieve the reversal endpoint of the skin fibrosis (assessed by modified Rodnan skin score), results for the secondary endpoint (predicted forced vital capacity) indicated that the treatment might preserve the lung function in persons with early involvement.
In addition, it also seems likely that combining different immunotherapies in treating scleroderma may be beneficial for patients. In a murine model, combined CD47 and IL-6 blockade reversed skin fibrosis and led to rapid elimination of ectopically transplanted scleroderma cells (Lerbs et al. 2020).
Rheumatoid arthritis
Rheumatoid arthritis, an autoimmune inflammatory disease, is a long-term condition that is characterized by pain, swelling and stiffness of the affected joints. In addition to immune cells, activated mesenchymal cells actively contribute to pathological tissue repair of joints, leading to pannus formation by perpetuation of an autoimmune reaction (Schuster et al. 2021). In rheumatoid arthritis, fibroblast-like synoviocytes are the most common cell type at the pannus–cartilage junction. Similarly to activated fibroblasts/myofibroblasts, these synoviocytes in rheumatoid arthritis contribute to the joint destruction via production of cytokines, chemokines and matrix-degrading agents (Bustamante et al. 2017). Like other autoimmune diseases, rheumatoid arthritis is frequently accompanied by extra-articular manifestations, namely lung fibrosis, with numerous myofibroblasts present in fibrotic tissue (Popper et al. 2021).
Rheumatoid arthritis is frequently associated with the elevation of IL-6. Pleiotropic effects of IL-6 participate in the control of articular and extra-articular pathologies (Jarlborg and Gabay 2022). Although IL-6 is up-regulated and may result in the cytokine storm, it is relatively rare in these patients (Mehta et al. 2020). On the other hand, the progression of the autoimmune disease resulting in cachexia and severe psychiatric issues (depression and anxiety) is common. It was suggested that it is mechanistically due to the up-regulation of bioactive factors such as TNF-α, IL-1, IL-6, IL-17 and C-reactive protein (CRP) (Fakra and Marotte 2021; Chimenti et al. 2021; Ollewagen et al. 2021). This concept has been confirmed, as anti-IL-6 therapy (tocilizumab or sarilumab) exhibited good efficacy and tolerability in rheumatoid arthritis with poor response to conventional treatment options (Yip and Yim 2021). Importantly, administration of these drugs treats the affected joints, but also alleviates extra-articular manifestations occurring in the patients. Consequently, stabilization of lung fibrosis and reduction of cachexia were also reported (d’Alessandro et al. 2020; Patsalos et al. 2020).
Cancer
Tumours mimic the complex structure of physiological organs. Tumours are structured and tightly orchestrated ecosystems formed jointly by malignant and non-malignant cells (Lacina et al. 2018). The elements of the tumour stroma [cancer-associated fibroblasts (CAFs), endothelial and immune cells and ECM] are not only bystanders, but are active participants of oncogenic processes. Cells within the cancer ecosystem also communicate directly via intercellular contacts or indirectly by releasing paracrine products. In many aspects, the tumour stroma resembles granulation tissue. Indeed, the tissue microenvironment of wounds (Čoma et al. 2021) and autoimmune disorders share many general features and regulatory mechanisms with the tumour stroma. Furthermore, the dynamic cellular interplay involving numerous cell types of the immune system in inflammation can also be relevant in cancer. Not surprisingly, an almost identical set of bioactive molecules can be observed during wound healing and cancer growth/spreading.
Precise targeting of tumour cells has been a primary goal of oncological therapy for decades. More recently, the development of immune checkpoint inhibitors, a novel class of immunotherapy drugs, has heralded a new era in cancer therapy (Smetana et al. 2020a; Genova et al. 2022; Yi et al. 2022). This therapeutic targeting of the tumour-resident immune cells proved to be a remarkably successful tool to treat cancer. In 2018, the Nobel Prize was awarded to Tasuku Honjo and James Allison for their contribution to this strategy.
On the other hand, cancer-associated fibroblasts (CAFs), another essential component of the tissue microenvironment (TME), have been largely overlooked in current treatment protocols. CAFs represent a fundamental bioactive part in many tumours. CAFs stimulate migration and poor differentiation of cancer cells (Kolář et al. 2012; Trylcova et al. 2015; Jobe et al. 2016, 2018). On the other hand, CAFs may also suppress the tumour microenvironment by lowering hepatocyte growth factor production, resulting in reduced tumour size and formation of metastasis (Pallangyo et al. 2015). Although CAFs influence the biological properties of tumours and support the process of metastasis formation, specific targeting of CAFs has not yet been widely employed in clinics.
In many aspects, CAFs share multiple features of myofibroblasts and represent one of the most prominent non-malignant cell types of many tumours producing bioactive factors (Plzák et al. 2019). CAFs may arise from normal resident tissue fibroblasts (recruited and activated by malignant cells), similarly to myofibroblasts arising from normal dermal fibroblasts in a wound. However, CAFs can also be derived from a wider panel of potential precursors such as mesenchymal stem cells, adipocytes, stellate cells, circulating fibrocytes, pericytes, mesothelial cells and epithelial and endothelial cells following exposure to potent pro-inflammatory factors (Liu et al. 2019), including but not limited to IL-1β, TGF-β and platelet-derived growth factor (PDGF) (Vokurka et al. 2022).
So far, there is no specific marker to identify all CAF subpopulations available for research and/or diagnostic purposes. The effects seem to be mediated especially by IL-6, IL-8, CXCL-1, CXCL-8 and specific ECM components. Mounting evidence illustrates that CAFs are a very heterogeneous population of cells (Kalluri 2016). The expression of fibroblast markers is extremely heterogeneous and varies strongly between different CAF subpopulations. Therefore, for reliable definition and detection of CAFs, it is recommended to use a broad panel of markers including produced cytokines and ECM proteins. Furthermore, this panel should also consider the origin of fibroblasts and the type of cancer. SMA (Fig. 3) and fibroblast activation protein alpha (FAP) seem to be widely accepted and stable markers of CAFs, separating CAFs from the larger pool of fibroblasts present in the body (Nurmik et al. 2020; Vokurka et al. 2022).
In this context, tumours with unfavourable prognosis (e.g. metastatic seminoma) have demonstrated a notable increase in pro-inflammatory activity, including IL-6 signalling (Nestler et al. 2022). Moreover, the production of pro-inflammatory mediators by CAFs can be significantly stimulated by exosomes released by cancer cells (Strnadová et al. 2022), indicating synergistic effects. However, whether these mechanisms are suitable as targets for novel therapies has to be answered in further studies in the future.
Mediators of intercellular crosstalk of the cancer ecosystem can also enter blood vessels and reach distant tissues with the ability to modulate their functions. It has been shown that IL-6 and CXCL-8 produced by CAFs can thus be detected in the circulating fluids (e.g. serum), making them useful for diagnostic/prognostic purposes to monitor disease progression. This was confirmed on a clinical basis in patients suffering from cutaneous malignant melanoma as well as ovarian, squamous cell, breast and colorectal cancers (Xu et al. 2016; Kučera et al. 2019; Rezaei et al. 2019; Wang et al. 2021; Amer et al. 2021; Paccagnella et al. 2022). For example, in patients with breast cancer, increased levels of IL-4, IL-6, IL-8, IL-10, CCL-2 and IFN-γ are associated with lower survival rates (Paccagnella et al. 2022).
In line with this evidence, IL-6 and other inflammation-supporting factors have an essential role in generating a so-called premetastatic niche. These tissue domains offer a permissive microenvironment allowing circulating tumour cells to harbour and foster in the sensitive initial period before they gain full control over the new metastatic site (Kodet et al. 2020; Li et al. 2020). Secretion of IL-6 and premetastatic niche formation is controlled by exosomal miR-21. It was shown in an animal model that miR-21 silencing reduces the level of IL-6 and minimizes metastasis of colorectal cancer to the liver (Shao et al. 2018).
The terminal stage of malignant disease is frequently associated with cancer-related cachexia. It is explained as a systemic effect of pro-inflammatory factors such as IL-6, CXCL-8 and TNF-α in skeletal muscle, adipocytes and hepatocytes (Kasprzak 2021; Paval et al. 2022). In many aspects, cancer-associated cachexia seems to be similar to that of a cytokine storm described in several viral infections (including COVID-19). High levels of IL-6 and other cytokines lead to deterioration of patients’ organs and cause exhaustion (White 2017; Yehia et al. 2021). Interestingly, moderate physical exercise can prevent, at least partially, the cancer-induced trend of cachexia (Wood et al. 2022). The increased level of IL-6 also influences the hypothalamus–pituitary–adrenal axis linking cancer with depression (Jehn et al. 2010) and reduction of food intake (anorexia) (Molfino et al. 2017). We may conclude that enhanced levels of pro-inflammation factors are associated with more severe psychiatric symptoms and further deteriorate the emotional wellbeing of patients with cancer (Santoft et al. 2020).
COVID-19
COVID-19 is a pandemic respiratory infection caused by the SARS-CoV-2 coronavirus. It is frequently accompanied by severe bilateral pneumonia resulting in acute respiratory distress syndrome. It can be fatal for many patients, especially those suffering from comorbidities associated with advanced age. Lung damage and respiratory problems can persist even after recovering from the acute disease (also known as long COVID-19). In this context, cases of lung transplantation for respiratory failure due to COVID-19 were also reported (Aul et al. 2021; Hall et al. 2021).
Lung autopsy of individuals who died from COVID-19 revealed three main histopathological patterns (Valdebenito et al. 2021):
-
(A) Haemorrhage with minimal immune infiltration and extensive thrombus,
-
(B) Heavy immune infiltration without thrombus formation, and
-
(C) Combination of type 1 and 2 with proliferation of fibroblasts and development of fibrosis.
Pulmonary fibrosis is an immensely interesting and potentially fatal feature resulting from this disease. This fibrosis causes gas-exchange impairment, profoundly affecting the quality of some surviving patients’ lives. However, the pathophysiological background of the resulting fibrosis remains relatively poorly understood. We believe that this is a critical aspect, and it is worthy of closer analysis.
Mesenchymal stem cells (MSCs) are present in nearly all tissues/organs of the body. MSCs are multipotent stem cells with self-renewal capacity. MSCs can generate clonal populations with the ability of multilineage differentiation. Thus, MSCs play an essential role in the repair and generation of tissues (Kavianpour et al. 2020). Notably, MSCs possess broad immunoregulatory properties. This is possible via interactions with the innate and adaptive immune systems. MSCs can cause immune suppression of many processes. Indeed, MSCs can attenuate the cytokine storm in severe/critical COVID-19, as shown in several studies (Kaffash Farkhad et al. 2021).
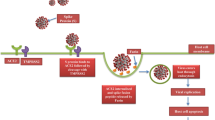
However, mammalian lungs, including human, contain heterogeneous subpopulations of mesenchymal cells that can modulate pulmonary fibrosis and functions (Liu et al. 2021). One of these populations are lung lipofibroblasts, cells with a remarkable ability to store lipid droplets. In collaboration with myofibroblasts, lipofibroblasts also participate in alveolar regeneration (Ushakumary et al. 2021). Under specific conditions such as hypoxia, lipofibroblasts can be extensively transformed into myofibroblasts and participate in the pathogenesis of lung fibrosis (Rehan and Torday 2003, 2014; Kruglikov and Scherer 2020). This process seems to be regulated by IL-6 and TGF-β (Fig. 4). Of note, it was reported that metformin alters the transition of lipofibroblasts into myofibroblasts. There is also evidence that this widely used oral antidiabetic drug minimizes lung fibrosis progression (Kheirollahi et al. 2019). Following viral injury of the lung alveoli and blood vessels, TGF-β and IL-6 initiate transformation of resident fibroblasts into myofibroblasts (Fig. 4). Moreover, these pro-inflammatory cytokines also induce EMT, EndMT and generation of myofibroblasts (Giacomelli et al. 2021). Of note, circulating fibrocytes (bone marrow-derived cells) were also suggested as a population potentially participating in myofibroblast induction (Ghanem et al. 2021).
Comparative analysis of a lung abscess (a1–g1) and lungs destructed by COVID-19 (a2–g2). The structure of the lungs is seriously altered in patients suffering from both diseases, as visible in the figures depicting haematoxylin and eosin staining (a1, a2). Negative control using isotype control antibodies (b1, b2) is included to confirm specificity of the following immunohistochemical reactions using horse radish peroxidase (HRP)-tagged antibodies and AEC (red) substrate. Expression of IL-6 was significantly lower in the lungs of the patient without COVID-19 (c1) than in the lungs of the patient with COVID-19 (c1). A similar trend was observed for the expression of TGF-β1 (d1, d2), type I collagen (e1, e2) and fibronectin (f1, f2). While in the non-COVID lungs SMA expression was limited to the smooth muscle cells in the wall of vessels and bronchiole (g1), numerous SMA-positive myofibroblasts were found in the COVID-19 lungs (g2). The presence of type I collagen in peribronchial fibrous tissue (h1) and SMA in vessels (h2) was visualized in the positive control confirming reactivity of primary antibodies. Nuclei were counterstained with Gill’s haematoxylin (scale bar, 100 µm)
The role of mesenchymal cells in fatal COVID-associated pneumonia is supported by recent data obtained using single-cell sequencing techniques. Analysis of tissue samples from affected lungs demonstrated, as expected, a broad spectrum of significantly activated infiltrating immune cells (such as macrophages). However, microscopic analysis also revealed a surprisingly large proportion of fibroblasts and myofibroblasts (Delorey et al. 2021; Wendisch et al. 2021). These studies also highlighted a remarkably limited ability of lung alveoli to self-renew following viral infection, such as infrequent local hyperplasia of alveolar type II cells.
It is known that SARS-CoV-2 enters the cells via angiotensin-converting enzyme 2 (ACE2) (the spike protein of the virus binds to ACE2) on the cell surface. ACE2 is expressed mainly on alveolar type II cells, making the lungs the primary target for the infection (Zou et al. 2020). ACE2 and transmembrane serine protease 2 (TMPRSS2) mediate virus internalization. These molecules are co-expressed in type II pneumocytes, ileal absorptive enterocytes and nasal goblet secretory cells (Ziegler et al. 2020). SARS-CoV-2 prefers activation by TMPRSS2, but if the target cells express low levels of TMPRSS2 or if the virus–ACE2 complex does not encounter TMPRSS2, the complex can be internalized through clathrin-mediated endocytosis (Karthika et al. 2021). Although type I alveolar cells are not primarily targeted by SARS-CoV-2, they develop from the type II cells. Thus, the type I pneumocyte population can be depleted after type II cells are destroyed owing to the viral cytopathic effect. Type II cells infected by SARS-CoV-2 produce high levels of IL-6 (Gajewski et al. 2021). The massive infection and subsequent inflammation-induced swelling also increase mechanic tension that stimulates production of TGF-β by type II pneumocytes. The increased mechanical stress facilitates the transition of fibroblasts/lipofibroblasts into myofibroblasts. This process is of critical importance for respiratory failure and development of pulmonary fibrosis (Wu et al. 2020). In combination with an increase in the fibroblast number and consequent ECM deposition (rich in fibronectin and collagen), the risk of developing severe/critical lung injury resulting in fibrosis and even death increases (Fig. 4) (Bridges et al. 2022).
It has been well demonstrated that the hyperactivation of the immune system accompanied by the enormous increase in the production of bioactive factors such as a panel of cytokines including IL-6, chemokines and growth factors leads to cytokine storm and is closely related to the severity of COVID-19 disease (Pedersen and Ho 2020; Smetana and Brábek 2020). Although understanding the pathogenesis of cytokine storm allows us to unravel risk factors for the condition and substantiates novel therapeutic strategies, evidence disputing the entire concept of cytokine storm has also been presented. Indeed, exceptions to this scenario are possible, as immunological responses strictly reflect the setting of an individual immune system. Indeed, some patients with severe COVID-19 exhibited no significant elevation of IL-6 (Wu et al. 2021). However, there is robust evidence that the severity of the COVID-19 disease – namely the level of lung damage – can be monitored via the detection of inflammatory markers in the serum, i.e. IL-6, CRP, procalcitonin and TNF-α represent the most reliable markers of the disease progression (Parimoo et al. 2021; Mardani et al. 2022). IL-6 has broad systemic effects and alters the metabolism, including age/cancer-related muscle wasting and cachexia (Pettersen et al. 2017).
Last but not least, research has also pointed to sex differences in the course of COVID-19 infection, since most countries showed disproportionately higher deaths and higher case fatality rates among men (Dehingia and Raj 2021). Sex differences refer to the biological attributes, including hormonal, immune and inflammatory responses to infection, that potentially influence the severity and outcomes of the infection (Ya’qoub et al. 2021). Oestrogens promote both innate and adaptive immune responses, potentially leading to faster clearance of pathogens, less severe symptoms in women and a more robust immune response to vaccines (Takahashi and Iwasaki 2021). Moreover, oestrogens reduce the risk of immune system hyperactivation during cytokine storms and the expression of ACE2 necessary for SARS-CoV-2 internalization. Therefore, oestrogens can protect women against serious disease progression (leading to lower mortality of women) (Abramenko et al. 2021).
Role of IL-6 and other inflammation-supporting factors
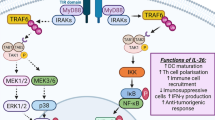
Our previous papers deal in detail with the mechanisms and cellular sources of the pleiotropic cytokine IL-6 (Lacina et al. 2019, 2021; Brábek et al. 2020; Čoma et al. 2021; Španko et al. 2021). Almost all cells in the human body can produce IL-6. The signalling is initiated via a heterodimeric complex consisting of IL-6 α-receptor (IL-6R) and signal-transducing β-subunit glycoprotein 130 (gp130) (Wolf et al. 2014). IL-6R may be found in soluble and membrane-bound forms, which allows distinction between IL-6 classic signalling (via the membrane-anchored IL-6R) and IL-6 trans-signalling via the soluble IL-6R (sIL-6R) (Rose-John 2012). Evidence suggests that IL-6 signalling via sIL-6R induces pro-inflammatory activity. In contrast, the anti-inflammatory activity of IL-6 is mediated via classic signalling. Therefore, specific inhibition of the sIL-6R pathway presents a valuable option for treating inflammatory diseases (Jones et al. 2011).
For example, a lack of IL-6 (and tumour necrosis factor receptor-1) results in more severe impairment of the innate immune response. IL-6 also stimulates proliferation of many cells and thus is essential for proper wound healing (Albrecht et al. 2016). An imbalance of pro-/anti-inflammatory cytokines may interfere with normal healing, whereas sustained elevated levels of IL-6 may lead to various complications (Rosa et al. 2008). Similarly, high levels of IL-6 are typical of autoimmune diseases such as rheumatoid arthritis or SSc. The cytokine also stimulates proliferation as well as migration/spreading of malignant cells, and induces low differentiation, which is an important hallmark of cancer resistance to conventional therapy (Jobe et al. 2016, 2018).
Why has IL-6 attracted so much attention in recent years? As we face population ageing in Western countries, the incidence of malignant and rheumatic diseases increases (Smetana et al. 2016; Iorio et al. 2021). Ageing also affects the immune system, referred to as immunosenescence. The altered setting of human immune response in the elderly is associated with systemic low-grade chronic inflammation, a phenomenon termed inflammaging (Franceschi et al. 2018; Brábek et al. 2020). It coincides with the accumulation of acquired mutations, partially due to the reduced gene repair capacity (Smetana et al. 2016) or long-term exposure to oxidative stress present in our environment (Bauer and de la Fuente 2016). According to a broadly accepted concept, inflammaging represents age-dependent deregulation of immune functions. It reduces adaptive immunity and is characterized by reduction of the immune response. It is also accompanied by up-regulation of pro-inflammatory cytokines, especially IL-6 and TNF-α. This mechanism may be related to numerous age-related health issues, including susceptibility to develop a more severe course of COVID-19 (Barbé-Tuana et al. 2020; Santoro et al. 2021). It has been well documented that individuals over 60–65 years of age are at a higher risk of COVID-19-related mortality. The concept of inflammaging is well supported by the immune response in survivors of childhood cancer who had received chemotherapy and/or radiation therapy as a consequence of premature ageing (Rossi et al. 2021). Children accumulate senescent cells, exhibit numerous DNA mutations and extensively produce reactive oxygen species that do not match their age. This may result in premature failure of vital organs (Rossi et al. 2021). Also typical of inflammaging is reshaping of the cytokine expression profile, with progressive tendency towards an inflammation-supporting phenotype (up-regulation of CRP, IFN-γ, IL-1, IL-6, IL-8, IL-12, TNF-α) (Rea et al. 2018).
Among these factors, IL-1, IL-6 and TNF-α remarkably support cancer growth and spreading (Jobe et al. 2016; Bleve et al. 2022; Strnadová et al. 2022). IL-6 represents a direct link between autoimmune disorders, cancer and age-related diseases, thus offering an attractive therapeutic target (Iorio et al. 2021). From this perspective, repurposing already available drugs for other clinical applications seems to be a highly rational approach. Therapeutic blocking of IL-6 signalling available for arthritis treatment can thus be an inspiring and readily achievable strategy for cancer therapy as well (Španko et al. 2021). However, from recently limited clinical experience, it seems that blocking of IL-6 is not sufficient to treat tumours. However, on the basis of experimental data, we can hypothesise that simultaneous blocking of other factors might be more promising, e.g. simultaneous inhibition of IL-6 and IL-8 (Jobe et al. 2016; Plzák et al. 2019; Zhang et al. 2022). For example, the combination of bazedoxifene (gp130 inhibitor) and paclitaxel seems to be potentially suitable for the therapy of ovarian cancer (Park et al. 2022).
It has been shown that the process of inflammaging can be promoted by external factors. For instance, chronic infection induced by cytomegalovirus (CMV) acts in a synergic manner, accelerating the natural process of inflammaging (Bauer and de la Fuente 2016). CMV infection together with immunosenescence and inflammaging contributes to hypersecretion of inflammation-supporting cytokines, resulting in a hyperinflammatory syndrome/cytokine storm with a severe-to-fatal course of COVID-19 in the elderly (Müller and di Benedetto 2021). The mediators of inflammation, such as IL-6 and TNF-α, also activate blood platelets, resulting in abnormal blood clotting – another severe complication of COVID-19 (Xu et al. 2021). To conclude this section, IL-6 has a central regulatory role in numerous physiological and pathological processes and thus represents an immensely promising target for therapeutic interventions.
The therapeutic consequences of COVID-19
Targeting the IL-6 signalling represents a plausible therapeutic strategy for several autoimmune diseases and cancers (Brábek et al. 2020; Španko et al. 2021) (Fig. 5). In recent years affected by the COVID-19 pandemic, the number of potential indications of this strategy has increased. The European Medicines Agency recently approved anti-IL-6 receptor monoclonal antibody tocilizumab to treat patients with severe and critical forms of COVID-19. The drug is recommended for patients with COVID-19 who progress to respiratory failure within 72 h after admission to hospital despite corticosteroid treatment. The guidelines for COVID-19 therapy also include sarilumab, which can be used instead of tocilizumab in case of its shortage.
Interleukin-6 (IL-6) signalling can be therapeutically regulated at several checkpoints. (1) The production of IL-6 can be diminished (e.g. using curcumin). (2) Once released, the bioavailability of IL-6 can be diminished by neutralizing antibodies (mAb) (e.g. siltuximab). (3) Another therapeutic approach can be based on targeting the interleukin-6 receptor (IL-6R) and/or its soluble form (sIL-6R) (e.g. tocilizumab, sarilumab). This can prevent IL-6R from binding to the cytokine IL-6 and formation of the active cytokine–receptor complex. Alternatively, antibodies or small-molecule inhibitors against the receptor or signal transducer gp130 can prevent the binding of the activated complex (IL-6/IL-6R or IL-6/sIL-6R) to signal transducer glycoprotein gp130 (e.g. bazedoxifene). (4) Antibodies raised against epitopes of signal transducer gp130 can also prevent the binding of the activated complexes (IL-6/IL-6R or IL-6/sIL-6R) (e.g. B-R3). (5) The soluble form of the signal transducer molecule (sgp130) binds the active complex of IL-6/sIL-6R (e.g. olamkicept). This has an inhibitory effect on trans-signalling by reduction of available sIL-6R. It can also help to sequestrate free IL-6. (6) Inhibitors of gp130 kinase activity prevent phosphorylation (P) of downstream signalling molecules (JAKs, STATs, PI3K, MAPK) (e.g. baricitinib), with their consequent translocation to the nucleus, where they regulate target gene transcription. Examples of drugs were selected from Španko et al. (2021)
Preliminary data from a single-centre study on 22 patients with COVID-19 performed at the Department of Infectious Diseases, First Faculty of Medicine, Military University Hospital Prague and Charles University, confirmed the positive effect observed in both tocilizumab-treated (n = 18) and sarilumab-treated (n = 4) groups of severe and critical forms of COVID-19. Only two fatal cases were recorded among the included sample of patients (n = 22), indicating in-hospital mortality slightly below 10%, which is lower than that reported in the first and second waves of COVID-19 (Gray et al. 2021; Martinez-Guerra et al. 2022) and correlates well with published data (Gupta et al. 2022).
The approval of EMA has opened a new horizon for anti-COVID-19 therapy. Perhaps it will also be soon translated to innovative therapeutic approaches to other viral infections accompanied by a cytokine storm. Potential therapeutic strategies (including low-molecular-weight inhibitors) can regulate cytokine production, block the active site of IL-6 binding, inhibit IL-6 receptor complex activity and/or block intracellular signalling (Kang et al. 2019). These drugs were mainly designed to treat autoimmune diseases (e.g. rheumatoid arthritis) or proposed for experimental cancer therapy; some drugs are already under investigation in several clinical trials in head and neck cancer, as reviewed in our recent article (Španko et al. 2021). Therefore, their use as new anti-COVID-19 therapeutics can be expected, but immediate clinical application warrants further careful evaluation.
As presented previously (Abramenko et al. 2021), natural oestrogens and some other oestrogen receptor modulators have improved the outcome of severe COVID-19, also inhibiting the interaction of IL-6 with its receptor. This mechanism reduces the risk of developing the cytokine storm. Some of these molecules, such as bazedoxifene or raloxifene, recognize the IL-6 receptor complex by interaction with gp130 and thus reduce the ability to bind IL-6. Both agents also recognize the viral spike protein and minimize virus entry to the target cells or interact with the viral main or papain-like protease inhibiting virus proliferation (Song et al. 2020; Brábek et al. 2020; Abramenko et al. 2021). This makes these drugs excellent candidates for repurposing. Numerous reports have indicated several candidate drugs (even broadly available) for COVID-19 treatment based on the inhibition of sIL-6 signalling. For example, anti-cholesterol medication lovastatin can reduce the level of IL-6. Indeed, it was reported that the COVID-19-related mortality of patients on statin treatment was significantly reduced (Karampoor et al. 2021).
Conclusion
The wound healing of various tissues is a precisely controlled process regulated by evolutionarily conserved signalling pathways. In the present review, we compared the general aspects of inflammatory/immune responses and regulation of this process in wounds, tumours, selected autoimmunity disorders and COVID-19 (Table 1).
We aimed to underscore the conceptual hypothesis of transition of the chronic course of a pathological healing-like process into an inflammation-resolving status that is beneficial for the patient. Specific regulation of inflammation occurs via controlled release of particular sets of cytokines/chemokines and immune cell populations, i.e. with factors critically relevant to health maintenance. The damage observed in autoimmune diseases develops owing to the failure of regulation and tolerance, resulting in a sustained, long-lasting course of inflammation with detrimental structural and functional effects on the whole organism. However, an acute hyperactive destructive immune over-response (cytokine storm) also results in deleterious effects, e.g. on the lungs of patients suffering from severe/critical COVID-19 disease. Furthermore, deregulated production of immune factors was associated with terminal cachexia in many patients with cancer. The inflammatory mediators are produced not only by recruited leucocytes, but also by local mesenchymal cells (including fibroblasts). Activated fibroblasts proliferate, transit to myofibroblasts and produce a huge amount of bioactive molecules regulating the inflammatory response. Both chronic and acute inflammatory over-reactions may consequently induce fibroproliferative changes. Fibrosis leads to deterioration of organ structure and impairment of organ function, thus presenting a devastating clinical issue. The context defines the outcome; Matthew 7:16 “You Will Know Them by Their Fruits”. Together, the role of stromal fibroblasts in the context of microenvironment regulations deserves serious attention, and their potential to become the targets of novel therapeutic concepts should be considered in multiple diseases.
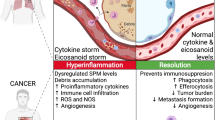
In summary, our review points out that organisms react in different pathological situations according to a uniform scenario using conservative pathways, as observed during wound healing, in selected autoimmune diseases, cancer and COVID-19. If poorly regulated, this can all be unfavourable and even fatal. All these situations are associated with inflammatory processes, including the involvement of both innate and adaptive immune responses. While re-epithelization of wounds negatively interferes with the activity/growth of the granulation tissue, in cancer, chronic inflammatory disorders or severe infections involving cytokine storm, a similar inhibitory feedback is not elicited (Fig. 6).
The diagram demonstrates the role of inflammation (with emphasis on IL-6) in wound healing, cancer and severe/critical COVID-19 infection. While wound re-epithelization arrests the activity of granulation tissue and reduces inflammation, this negative feedback fails to act in the case of cancer and severe/critical COVID-19, worsening the prognosis of patients
Therefore, detailed understanding of the mechanisms regulating wound healing and driving autoimmunity and molecular signalling pathways in the tumour ecosystem provides immensely valuable information for other clinically relevant applications as well. In particular, the COVID-19 pandemic allowed the development of a strategy to rapidly repurpose available drugs. Almost instantly, this offered various therapeutic options for the management of hyperactivation of the immune system (e.g. anti-IL-6 therapy). In line with this experience, similar approaches may also follow as novel therapies for other, seemingly unrelated diseases.
References
Abramenko N, Vellieux F, Tesařová P et al (2021) Estrogen receptor modulators in viral infections such as SARS−CoV−2: therapeutic consequences. Int J Mol Sci 22(12):6551. https://doi.org/10.3390/ijms22126551
Adamik B, Zimecki M, Właszczyk A, Kübler A (1997) Immunological status of septic and trauma patients. I. High tumor necrosis factor α serum levels in septic and trauma patients are not responsible for increased mortality; a prognostic value of serum interleukin 6. Arch Immunol Ther Exp 45(2–3):169–175 (PMID: 9597083)
Albrecht LJ, Tauber SC, Merres J et al (2016) Lack of proinflammatory cytokine interleukin-6 or tumor necrosis factor receptor-1 results in a failure of the innate immune response after bacterial meningitis. Mediators Inflamm. https://doi.org/10.1155/2016/7678542
Amer H, Kartikasari AER, Plebanski M (2021) Elevated interleukin-6 levels in the circulation and peritoneal fluid of patients with ovarian cancer as a potential diagnostic biomarker: a systematic review and meta-analysis. J Pers Med. https://doi.org/10.3390/JPM11121335
Aul DR, Gates DJ, Draper DA et al (2021) Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir Med. https://doi.org/10.1016/j.rmed.2021.106602
Avazi DO, Awasum AC, Hassan AZ et al (2019) Evaluation of levels of interleukin-6, interleukin-8 and some haematologic parameters of dogs with cutaneous wounds. Cytokine. https://doi.org/10.1016/j.cyto.2018.06.024
Barbé-Tuana F, Funchal G, Schmitz CRR et al (2020) The interplay between immunosenescence and age-related diseases. Semin Immunopathol 42(5):545–557. https://doi.org/10.1007/s00281-020-00806-z
Barleon B, Sozzani S, Zhou D et al (1996) Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87:3336–3343. https://doi.org/10.1182/BLOOD.V87.8.3336.BLOODJOURNAL8783336
Bauer ME, de la Fuente M (2016) The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech Ageing Dev 158:27–37. https://doi.org/10.1016/j.mad.2016.01.001
Bleve A, Motta F, Durante B et al (2022) Immunosenescence, inflammaging, and frailty: role of myeloid cells in age-related diseases. Clin Rev Allergy Immunol 1:1. https://doi.org/10.1007/S12016-021-08909-7
Bonner JC, Badgett A, Osornio-Vargas AR et al (1990) PDGF-stimulated fibroblast proliferation is enhanced synergistically by receptor-recognized alpha 2-macroglobulin. J Cell Physiol 145:1–8. https://doi.org/10.1002/JCP.1041450102
Borsky J, Veleminska J, Jurovčík M et al (2012) Successful early neonatal repair of cleft lip within first 8 days of life. Int J Pediatr Otorhinolaryngol 76:1616–1626. https://doi.org/10.1016/J.IJPORL.2012.07.031
Brábek J, Jakubek M, Vellieux F et al (2020) Interleukin-6: molecule in the intersection of cancer, ageing and COVID-19. Int J Mol Sci 21:1–25. https://doi.org/10.3390/IJMS21217937
Bridges JP, Vladar EK, Huang H, Mason RJ (2022) Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 77:203–209. https://doi.org/10.1136/THORAXJNL-2021-217561
Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M (2017) Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. https://doi.org/10.1186/S13075-017-1303-3
Cámara-Lemarroy CR, Guzmán-De La Garza FJ, Fernández-Garza NE (2010) Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. NeuroImmunoModulation 17:314–324. https://doi.org/10.1159/000292020
Chimenti MS, Fonti GL, Conigliaro P et al (2021) The burden of depressive disorders in musculoskeletal diseases: is there an association between mood and inflammation? Ann Gen Psychiatry. https://doi.org/10.1186/S12991-020-00322-2
Chitturi RT, Balasubramaniam AM, Parameswar RA et al (2015) The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J Int Oral Health 7:75 (PMID: 25878485; PMCID: PMC4385733)
Clark RA, McCOY GA, Folkvord JM, McPHERSON JM (1997) TGF-β1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol 170:69–80. https://doi.org/10.1002/(SICI)1097-4652(199701)170:1
Cobianchi S, de Cruz J, Navarro X (2014) Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp Neurol 255:1–11. https://doi.org/10.1016/J.EXPNEUROL.2014.02.008
Čoma M, Fröhlichová L, Urban L et al (2021) Molecular changes underlying hypertrophic scarring following burns involve specific deregulations at all wound healing stages (inflammation, proliferation and maturation). Int J Mol Sci 22:1–20. https://doi.org/10.3390/IJMS22020897
d’Alessandro M, Perillo F, Metella Refini R et al (2020) Efficacy of baricitinib in treating rheumatoid arthritis: modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int Immunopharmacol. https://doi.org/10.1016/J.INTIMP.2020.106748
de Oliveira S, Rosowski EE, Huttenlocher A (2016) Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16:378–391. https://doi.org/10.1038/NRI.2016.49
Declercq J, van Damme KFA, de Leeuw E et al (2021) Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet Respir Med 9:1427–1438. https://doi.org/10.1016/S2213-2600(21)00377-5
Dehingia N, Raj A (2021) Sex differences in COVID-19 case fatality: do we know enough? Lancet Glob Health 9:e14–e15. https://doi.org/10.1016/S2214-109X(20)30464-2
Delorey TM, Ziegler CGK, Heimberg G et al (2021) A single-cell and spatial atlas of autopsy tissues reveals pathology and cellular targets of SARS-CoV-2. bioRxiv. https://doi.org/10.1101/2021.02.25.430130
Distler O, Assassi S, Cottin V et al (2020) Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur Respir J. https://doi.org/10.1183/13993003.02026-2019
Dubový P, Hradilová-Svíženská I, Klusáková I et al (2019) Interleukin-6 contributes to initiation of neuronal regeneration program in the remote dorsal root ganglia neurons after sciatic nerve injury. Histochem Cell Biol 152:109–117. https://doi.org/10.1007/S00418-019-01779-3
Dubový P, Hradilová-Svíženská I, Brázda V, Joukal M (2021) Toll-like receptor 9-mediated neuronal innate immune reaction is associated with initiating a pro-regenerative state in neurons of the dorsal root ganglia non-associated with sciatic nerve lesion. Int J Mol Sci. https://doi.org/10.3390/IJMS22147446
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. https://doi.org/10.1056/NEJM198612253152606
Dvořánková B, Szabo P, Lacina L et al (2011) Human galectins induce conversion of dermal fibroblasts into myofibroblasts and production of extracellular matrix: potential application in tissue engineering and wound repair. Cells Tissues Organs 194:469–480. https://doi.org/10.1159/000324864
Eelen G, Treps L, Li X, Carmeliet P (2020) Basic and therapeutic aspects of angiogenesis updated. Circ Res 127:310–329. https://doi.org/10.1161/CIRCRESAHA.120.316851
Ehrlich P (1900) Croonian lecture—on immunity with special reference to cell life. Proc R Soc Lond 66:424–448. https://doi.org/10.1098/RSPL.1899.0121
Ellis S, Lin EJ, Tartar D (2018) Immunology of wound healing. Curr Dermatol Rep 7:350–358. https://doi.org/10.1007/S13671-018-0234-9
Epstein Shochet G, Brook E, Bardenstein-Wald B, Shitrit D (2020) TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. https://doi.org/10.1186/S12931-020-1319-0
Evans RA, Tian YC, Steadman R, Phillips AO (2003) TGF-β1-mediated fibroblast-myofibroblast terminal differentiation – the role of Smad proteins. Exp Cell Res 282:90–100. https://doi.org/10.1016/S0014-4827(02)00015-0
Fakra E, Marotte H (2021) Rheumatoid arthritis and depression. Joint Bone Spine. https://doi.org/10.1016/J.JBSPIN.2021.105200
Ferreli C, Gasparini G, Parodi A et al (2017) Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol 53:306–336. https://doi.org/10.1007/S12016-017-8625-4
Fertala J, Rivlin M, Wang ML et al (2020) Collagen-rich deposit formation in the sciatic nerve after injury and surgical repair: a study of collagen-producing cells in a rabbit model. Brain Behav. https://doi.org/10.1002/BRB3.1802
Franceschi C, Garagnani P, Parini P et al (2018) Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590. https://doi.org/10.1038/S41574-018-0059-4
Gajewski T, Rouhani S, Trujillo J et al (2021) Severe COVID-19 infection is associated with aberrant cytokine production by infected lung epithelial cells rather than by systemic immune dysfunction. Res Sq. https://doi.org/10.21203/RS.3.RS-1083825/V1
Genova C, Dellepiane C, Carrega P et al (2022) Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front Immunol. https://doi.org/10.3389/FIMMU.2021.799455
Ghanem M, Homps-Legrand M, Garnier M et al (2021) Blood fibrocytes are associated with severity and prognosis in COVID-19 pneumonia. Am J Physiol Lung Cell Mol Physiol 321:L847–L858. https://doi.org/10.1152/AJPLUNG.00105.2021
Giacomelli C, Piccarducci R, Marchetti L et al (2021) Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients. Biochem Pharmacol. https://doi.org/10.1016/J.BCP.2021.114812
Gray WK, Navaratnam AV, Day J et al (2021) COVID-19 hospital activity and in-hospital mortality during the first and second waves of the pandemic in England: an observational study. Thorax. https://doi.org/10.1136/THORAXJNL-2021-218025
Grazul-Bilska AT, Luthra G, Reynolds LP et al (2002) Effects of basic fibroblast growth factor (FGF-2) on proliferation of human skin fibroblasts in type II diabetes mellitus. Exp Clin Endocrinol Diabetes 110:176–181. https://doi.org/10.1055/S-2002-32149
Griffin MF, desJardins-Park HE, Mascharak S, et al (2020) Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis Model Mech. https://doi.org/10.1242/DMM.044164
Gupta S, Padappayil RP, Bansal A et al (2022) Tocilizumab in patients hospitalized with COVID-19 pneumonia: systematic review and meta-analysis of randomized controlled trials. J Investig Med 70:55–60. https://doi.org/10.1136/JIM-2021-002001
Hall DJ, Schulte JJ, Lewis EE et al (2021) Successful lung transplantation for severe post COVID-19 pulmonary fibrosis. Ann Thorac Surg. https://doi.org/10.1016/J.ATHORACSUR.2021.10.004
Henshaw FR, Dewsbury LS, Lim CK, Steiner GZ (2021) The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in vivo studies. Cannabis Cannabinoid Res 6:177–195. https://doi.org/10.1089/CAN.2020.0105
Hinz B (2016) The role of myofibroblasts in wound healing. Curr Res Transl Med 64:171–177. https://doi.org/10.1016/J.RETRAM.2016.09.003
Hirota H, Kiyama H, Kishimoto T, Taga T (1996) Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med 183:2627–2634. https://doi.org/10.1084/JEM.183.6.2627
Hung CF, Rohani MG, Lee S Soon, et al (2013) Role of IGF-1 pathway in lung fibroblast activation. Respir Res https://doi.org/10.1186/1465-9921-14-102
Iorio GC, Ammendolia A, Marotta N et al (2021) A bond between rheumatic diseases and cancer in the elderly: the interleukin-6 pathway. Int J Rheum Dis 24:1317–1320. https://doi.org/10.1111/1756-185X.14194
Jarlborg M, Gabay C (2022) Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine. https://doi.org/10.1016/J.CYTO.2021.155742
Jehn CF, Kühnhardt D, Bartholomae A et al (2010) Association of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancer. Integr Cancer Ther 9:270–275. https://doi.org/10.1177/1534735410370036
Jobe NP, Rösel D, Dvořánková B et al (2016) Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem Cell Biol 146:205–217. https://doi.org/10.1007/S00418-016-1433-8
Jobe NP, Živicová V, Mifková A et al (2018) Fibroblasts potentiate melanoma cells in vitro invasiveness induced by UV-irradiated keratinocytes. Histochem Cell Biol 149:503–516. https://doi.org/10.1007/S00418-018-1650-4
Jones SA, Scheller J, Rose-John S (2011) Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest 121:3375–3383. https://doi.org/10.1172/JCI57158
Kaffash Farkhad N, Reihani H, Sedaghat A et al (2021) Are mesenchymal stem cells able to manage cytokine storm in COVID-19 patients? A review of recent studies. Regen Ther 18:152–160. https://doi.org/10.1016/J.RETH.2021.05.007
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598. https://doi.org/10.1038/nrc.2016.73
Kang S, Tanaka T, Narazaki M, Kishimoto T (2019) Targeting interleukin-6 signaling in clinic. Immunity 50:1007–1023. https://doi.org/10.1016/J.IMMUNI.2019.03.026
Karampoor S, Hesamizadeh K, Shams Z et al (2021) The role of lovastatin in the attenuation of COVID-19. Int Immunopharmacol. https://doi.org/10.1016/J.INTIMP.2021.108192
Karthika T, Joseph J, Akshay Das VR et al (2021) SARS-CoV-2 cellular entry is independent of the ACE2 cytoplasmic domain signaling. Cells. https://doi.org/10.3390/CELLS10071814
Kasprzak A (2021) The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci 22:1–34. https://doi.org/10.3390/IJMS22041565
Katenkamp D, Stiller D (1978) Ultrastructure of perineurial cells during peripheral nerve regeneration. Electron microscopical investigations on the so-called amputation neuroma. Exp Pathol (Jena) 16:5–15. https://doi.org/10.1016/S0014-4908(78)80002-7
Kavianpour M, Saleh M, Verdi J (2020) The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Res Ther. https://doi.org/10.1186/S13287-020-01849-7
Khanna D, Lin CJF, Furst DE et al (2020) Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 8:963–974. https://doi.org/10.1016/S2213-2600(20)30318-0
Kheirollahi V, Wasnick RM, Biasin V et al (2019) Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. https://doi.org/10.1038/S41467-019-10839-0
Kodet O, Kučera J, Strnadová K et al (2020) Cutaneous melanoma dissemination is dependent on the malignant cell properties and factors of intercellular crosstalk in the cancer microenvironment (review). Int J Oncol 57:619–630. https://doi.org/10.3892/IJO.2020.5090
Kolář M, Szabo P, Dvořánková B et al (2012) Upregulation of IL-6, IL-8 and CXCL-1 production in dermal fibroblasts by normal/malignant epithelial cells in vitro: Immunohistochemical and transcriptomic analyses. Biol Cell 104:738–751. https://doi.org/10.1111/BOC.201200018
Kruglikov IL, Scherer PE (2020) The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity 28:1187–1190. https://doi.org/10.1002/OBY.22856
Kučera J, Strnadová K, Dvořánková B et al (2019) Serum proteomic analysis of melanoma patients with immunohistochemical profiling of primary melanomas and cultured cells: pilot study. Oncol Rep 42:1793–1804. https://doi.org/10.3892/OR.2019.7319
Lacina L, Kodet O, Dvořánková B et al (2018) Ecology of melanoma cell. Histol Histopathol 33:247–254. https://doi.org/10.14670/HH-11-926
Lacina L, Brábek J, Král V et al (2019) Interleukin-6: a molecule with complex biological impact in cancer. Histol Histopathol 34:125–136. https://doi.org/10.14670/HH-18-033
Lacina L, Brábek J, Fingerhutová Š et al (2021) Pediatric inflammatory multisystem syndrome (PIMS)—potential role for cytokines such Is IL-6. Physiol Res 70:153–159. https://doi.org/10.33549/PHYSIOLRES.934673
Lamontagne F, Agoritsas T, MacDonald H et al (2020) A living WHO guideline on drugs for COVID-19. BMJ. https://doi.org/10.1136/BMJ.M3379
Landén NX, Li D, Ståhle M (2016) Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 73:3861–3885. https://doi.org/10.1007/S00018-016-2268-0
Leask A (2013) Focal adhesion kinase: a key mediator of transforming growth factor beta signaling in fibroblasts. Adv Wound Care 2:247–249. https://doi.org/10.1089/WOUND.2012.0363
Lerbs T, Cui L, King ME et al (2020) CD47 prevents the elimination of diseased fibroblasts in scleroderma. JCI Insight. https://doi.org/10.1172/JCI.INSIGHT.140458
Li B, Wang JHC (2011) Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 20:108–120. https://doi.org/10.1016/J.JTV.2009.11.004
Li H, Yao Z, He W et al (2016) P311 induces the transdifferentiation of epidermal stem cells to myofibroblast-like cells by stimulating transforming growth factor β1 expression. Stem Cell Res Ther 7:1–17. https://doi.org/10.1186/S13287-016-0421-1
Li R, Wen A, Lin J (2020) Pro-inflammatory cytokines in the formation of the pre-metastatic niche. Cancers 12:1–16. https://doi.org/10.3390/CANCERS12123752
Li X, Guan Y, Li C et al (2022) Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res Ther. https://doi.org/10.1186/S13287-021-02690-2
Lindborg JA, Niemi JP, Howarth MA et al (2018) Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation. https://doi.org/10.1186/S12974-018-1222-5
Liu T, Han C, Wang S et al (2019) Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. https://doi.org/10.1186/S13045-019-0770-1
Liu X, Rowan SC, Liang J et al (2021) Categorization of lung mesenchymal cells in development and fibrosis. iScience. https://doi.org/10.1016/J.ISCI.2021.102551
Ma Y, Dong L, Zhou D et al (2019) Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med 23:2822–2835. https://doi.org/10.1111/JCMM.14190
Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J (2021) Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol 30:132–145. https://doi.org/10.1111/EXD.14243
Majidpoor J, Mortezaee K (2022) Interleukin-6 in SARS-CoV-2 induced disease: interactions and therapeutic applications. Biomed Pharmacother. https://doi.org/10.1016/J.BIOPHA.2021.112419
Mardani R, Namavar M, Ghorbi E et al (2022) Association between serum inflammatory parameters and the disease severity in COVID-19 patients. J Clin Lab Anal. https://doi.org/10.1002/JCLA.24162
Martinez-Guerra BA, Gonzalez-Lara MF, Roman-Montes CM et al (2022) Outcomes of patients with severe and critical COVID-19 treated with dexamethasone: a prospective cohort study. Emerg Microbes Infect 11:50–59. https://doi.org/10.1080/22221751.2021.2011619
Mateu R, Ẑivicová V, Krejí ED et al (2016) Functional differences between neonatal and adult fibroblasts and keratinocytes: donor age affects epithelial-mesenchymal crosstalk in vitro. Int J Mol Med 38:1063–1074. https://doi.org/10.3892/IJMM.2016.2706
Mehta P, Cron RQ, Hartwell J et al (2020) Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol 2:e358–e367. https://doi.org/10.1016/S2665-9913(20)30096-5
Molfino A, Iannace A, Colaiacomo MC et al (2017) Cancer anorexia: hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle 8:40–47. https://doi.org/10.1002/JCSM.12156
Mulder PPG, Vlig M, Boekema BKHL et al (2021) Persistent systemic inflammation in patients with severe burn injury is accompanied by influx of immature neutrophils and shifts in T cell subsets and cytokine profiles. Front Immunol. https://doi.org/10.3389/FIMMU.2020.621222
Müller L, di Benedetto S (2021) How immunosenescence and inflammaging may contribute to hyperinflammatory syndrome in COVID-19. Int J Mol Sci. https://doi.org/10.3390/IJMS222212539
Nestler T, Dalvi P, Haidl F et al (2022) Transcriptome analysis reveals upregulation of immune response pathways at the invasive tumour front of metastatic seminoma germ cell tumours. Br J Cancer 126:937–947. https://doi.org/10.1038/S41416-021-01621-5
Niemi JP, DeFrancesco-Lisowitz A, Cregg JM et al (2016) Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp Neurol 275(Pt 1):25–37. https://doi.org/10.1016/J.EXPNEUROL.2015.09.018
Nurmik M, Ullmann P, Rodriguez F et al (2020) In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer 146:895–905. https://doi.org/10.1002/IJC.32193
Nyika DT, Khumalo NP, Bayat A (2022) Genetics and epigenetics of keloids. Adv Wound Care 11:192–201. https://doi.org/10.1089/WOUND.2021.0094
Oliveira KMC, Pindur L, Han Z et al (2018) Time course of traumatic neuroma development. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0200548
Ollewagen T, Myburgh KH, van de Vyver M, Smith C (2021) Rheumatoid cachexia: the underappreciated role of myoblast, macrophage and fibroblast interplay in the skeletal muscle niche. J Biomed Sci. https://doi.org/10.1186/S12929-021-00714-W
Paccagnella M, Abbona A, Michelotti A et al (2022) Circulating cytokines in metastatic breast cancer patients select different prognostic groups and patients who might benefit from treatment beyond progression. Vaccines. https://doi.org/10.3390/VACCINES10010078
Pallangyo CK, Ziegler PK, Greten FR (2015) IKKβ acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J Exp Med 212:2253–2266. https://doi.org/10.1084/JEM.20150576
Pannell M, Labuz D, Celik M et al (2016) Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation. https://doi.org/10.1186/S12974-016-0735-Z
Parimoo A, Biswas A, Baitha U et al (2021) Dynamics of inflammatory markers in predicting mortality in COVID-19. Cureus. https://doi.org/10.7759/CUREUS.19080
Park SA, Kim LK, Park HM et al (2022) Inhibition of GP130/STAT3 and EMT by combined bazedoxifene and paclitaxel treatment in ovarian cancer. Oncol Rep. https://doi.org/10.3892/OR.2022.8263
Pastar I, Stojadinovic O, Yin NC et al (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care 3:445–464. https://doi.org/10.1089/WOUND.2013.0473
Patil S, Testarelli L (2021) Assessment of growth factors, cytokines, and cellular markers in saliva of patients with trigeminal neuralgia. Molecules. https://doi.org/10.3390/MOLECULES26102964
Patsalos O, Dalton B, Himmerich H (2020) Effects of IL-6 signaling pathway inhibition on weight and BMI: a systematic review and meta-analysis. Int J Mol Sci 21:1–13. https://doi.org/10.3390/IJMS21176290
Paval DR, Patton R, McDonald J et al (2022) A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/JCSM.12912
Pedersen SF, Ho YC (2020) SARS-CoV-2: a storm is raging. J Clin Invest 130:2202–2205. https://doi.org/10.1172/JCI137647
Pedroza M, Schneider DJ, Karmouty-Quintana H et al (2011) Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0022667
Pettersen K, Andersen S, Degen S et al (2017) Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep. https://doi.org/10.1038/S41598-017-02088-2
Piera-Velazquez S, Li Z, Jimenez SA (2011) Role of endothelial–mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 179:1074–1080. https://doi.org/10.1016/J.AJPATH.2011.06.001
Plzák J, Bouček J, Bandúrová V et al (2019) The head and neck squamous cell carcinoma microenvironment as a potential target for cancer therapy. Cancers. https://doi.org/10.3390/CANCERS11040440
Pollini M, Paladini F (2020) Bioinspired materials for wound healing application: the potential of silk fibroin. Materials 13:3361. https://doi.org/10.3390/MA13153361
Popper H, Stacher-Priehse E, Brcic L, Nerlich A (2021) Lung fibrosis in autoimmune diseases and hypersensitivity: how to separate these from idiopathic pulmonary fibrosis. Rheumatol Int. https://doi.org/10.1007/S00296-021-05002-2
Quan TE, Cowper SE, Bucala R (2006) The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 8:145–150. https://doi.org/10.1007/S11926-006-0055-X
Ramirez AM, Shen Z, Ritzenthaler JD, Roman J (2006) Myofibroblast transdifferentiation in obliterative bronchiolitis: TGF-β signaling through smad3-dependent and -independent pathways. Am J Transplant 6:2080–2088. https://doi.org/10.1111/J.1600-6143.2006.01430.X
Rao PN, Mainkar O, Bansal N et al (2021) Flavonoids in the treatment of neuropathic pain. Curr Pain Headache Rep. https://doi.org/10.1007/S11916-021-00959-Y
Rea IM, Gibson DS, McGilligan V et al (2018) Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. https://doi.org/10.3389/FIMMU.2018.00586
Rehan VK, Torday JS (2003) Hyperoxia augments pulmonary lipofibroblast-to-myofibroblast transdifferentiation. Cell Biochem Biophys 38:239–249. https://doi.org/10.1385/CBB:38:3:239
Rehan VK, Torday JS (2014) The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal 21:1893–1904. https://doi.org/10.1089/ARS.2013.5793
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49:35–43. https://doi.org/10.1159/000339613
Rezaei F, Mozaffari HR, Tavasoli J et al (2019) Evaluation of serum and salivary interleukin-6 and interleukin-8 levels in oral squamous cell carcinoma patients: systematic review and meta-analysis. J Interferon Cytokine Res 39:727–739. https://doi.org/10.1089/JIR.2019.0070
Rosa JS, Flores RL, Oliver SR et al (2008) Sustained IL-1alpha, IL-4, and IL-6 elevations following correction of hyperglycemia in children with type 1 diabetes mellitus. Pediatr Diabetes 9:9–16. https://doi.org/10.1111/J.1399-5448.2007.00243.X
Rose-John S (2012) IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 8:1237–1247. https://doi.org/10.7150/IJBS.4989
Rossi F, di Paola A, Pota E et al (2021) Biological aspects of inflamm-aging in childhood cancer survivors. Cancers. https://doi.org/10.3390/CANCERS13194933
Saikia P, Crabb JS, Dibbin LL et al (2020) Quantitative proteomic comparison of myofibroblasts derived from bone marrow and cornea. Sci Rep. https://doi.org/10.1038/S41598-020-73686-W
Santoft F, Hedman-Lagerlöf E, Salomonsson S et al (2020) Inflammatory cytokines in patients with common mental disorders treated with cognitive behavior therapy. Brain Behav Immun Health 3:100045. https://doi.org/10.1016/J.BBIH.2020.100045
Santoro A, Bientinesi E, Monti D (2021) Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. https://doi.org/10.1016/J.ARR.2021.101422
Schuster R, Rockel JS, Kapoor M, Hinz B (2021) The inflammatory speech of fibroblasts. Immunol Rev 302:126–146. https://doi.org/10.1111/IMR.12971
Shao Y, Chen T, Zheng X et al (2018) Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis 39:1368–1379. https://doi.org/10.1093/CARCIN/BGY115
Shima Y, Kuwahara Y, Murota H et al (2010) The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology 49:2408–2412. https://doi.org/10.1093/RHEUMATOLOGY/KEQ275
Smetana K, Brábek J (2020) Role of interleukin-6 in lung complications in patients with COVID-19: therapeutic implications. In Vivo 34:1589–1592. https://doi.org/10.21873/INVIVO.11947
Smetana K, Lacina L, Szabo P et al (2016) Ageing as an important risk factor for cancer. Anticancer Res 36:5009–5017. https://doi.org/10.21873/ANTICANRES.11069
Smetana K, Lacina L, Kodet O (2020a) Targeted therapies for melanoma. Cancers 12:1–4. https://doi.org/10.3390/CANCERS12092494
Smetana K, Rosel D, Brábek J (2020b) Raloxifene and bazedoxifene could be promising candidates for preventing the COVID-19 related cytokine storm, ARDS and mortality. In Vivo 34:3027–3028. https://doi.org/10.21873/INVIVO.12135
Song D, Yu W, Ren Y et al (2020) Discovery of bazedoxifene analogues targeting glycoprotein 130. Eur J Med Chem 199:112375. https://doi.org/10.1016/J.EJMECH.2020.112375
Španko M, Strnadová K, Pavlíček AJ et al (2021) IL-6 in the ecosystem of head and neck cancer: possible therapeutic perspectives. Int J Mol Sci. https://doi.org/10.3390/IJMS222011027
Strnadová K, Pfeiferová L, Přikryl P et al (2022) Exosomes produced by melanoma cells significantly influence the biological properties of normal and cancer-associated fibroblasts. Histochem Cell Biol 157:153–172. https://doi.org/10.1007/S00418-021-02052-2
Takahashi T, Iwasaki A (2021) Sex differences in immune responses. Science 371:347–348. https://doi.org/10.1126/SCIENCE.ABE7199
Te Lin Y, Chen JS, Wu MH et al (2015) Galectin-1 accelerates wound healing by regulating the neuropilin-1/Smad3/NOX4 pathway and ROS production in myofibroblasts. J Invest Dermatol 135:258–268. https://doi.org/10.1038/JID.2014.288
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial–mesenchymal transitions in development and disease. Cell 139:871–890. https://doi.org/10.1016/J.CELL.2009.11.007
Thulabandu V, Chen D, Atit RP (2018) Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol. https://doi.org/10.1002/WDEV.307
Tracy LE, Minasian RA, Caterson EJ (2016) Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care 5:119–136. https://doi.org/10.1089/WOUND.2014.0561
Trylcova J, Busek P, Smetana K et al (2015) Effect of cancer-associated fibroblasts on the migration of glioma cells in vitro. Tumour Biol 36:5873–5879. https://doi.org/10.1007/S13277-015-3259-8
Urch CE, Dickenson AH (2008) Neuropathic pain in cancer. Eur J Cancer 44:1091–1096. https://doi.org/10.1016/J.EJCA.2008.03.015
Ushakumary MG, Riccetti M, Perl AKT (2021) Resident interstitial lung fibroblasts and their role in alveolar stem cell niche development, homeostasis, injury, and regeneration. Stem Cells Transl Med 10:1021–1032. https://doi.org/10.1002/SCTM.20-0526
Valdebenito S, Bessis S, Annane D et al (2021) COVID-19 lung pathogenesis in SARS-CoV-2 autopsy cases. Front Immunol. https://doi.org/10.3389/FIMMU.2021.735922
van Caam A, Vonk M, van den Hoogen F et al (2018) Unraveling SSc pathophysiology; the myofibroblast. Front Immuno. https://doi.org/10.3389/FIMMU.2018.02452
van Praet JT, Smith V, Haspeslagh M et al (2011) Histopathological cutaneous alterations in systemic sclerosis: a clinicopathological study. Arthritis Res Ther. https://doi.org/10.1186/AR3267
Vokurka M, Lacina L, Brábek J et al (2022) Cancer-associated fibroblasts influence the biological properties of malignant tumours via paracrine secretion and exosome production. Int J Mol Sci. https://doi.org/10.3390/IJMS23020964
Vorstandlechner V, Laggner M, Kalinina P et al (2020) Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J 34:3677–3692. https://doi.org/10.1096/FJ.201902001RR
Wang ML, Rivlin M, Graham JG, Beredjiklian PK (2019) Peripheral nerve injury, scarring, and recovery. Connect Tissue Res 60:3–9. https://doi.org/10.1080/03008207.2018.1489381
Wang Y, Ramachandran V, Sui D et al (2021) Evaluation of plasma IL-6 in patients with melanoma as a prognostic and checkpoint immunotherapy predictive biomarker. J Invest Dermatol. https://doi.org/10.1016/J.JID.2021.12.012
Wendisch D, Dietrich O, Mari T et al (2021) SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 184:6243-6261.e27. https://doi.org/10.1016/J.CELL.2021.11.033
White JP (2017) IL-6, cancer and cachexia: metabolic dysfunction creates the perfect storm. Transl Cancer Res 6:S280–S285. https://doi.org/10.21037/TCR.2017.03.52
Wilgus TA (2020) Inflammation as an orchestrator of cutaneous scar formation: a review of the literature. Plast Aesthet Res. https://doi.org/10.20517/2347-9264.2020.150
Wofford KL, Shultz RB, Burrell JC, Cullen DK (2022) Neuroimmune interactions and immunoengineering strategies in peripheral nerve repair. Prog Neurobiol. https://doi.org/10.1016/J.PNEUROBIO.2021.102172
Wokalek H, Ruh H (1991) Time course of wound healing. J Biomater Appl 5:337–362. https://doi.org/10.1177/088532829100500405
Wolf J, Rose-John S, Garbers C (2014) Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 70:11–20. https://doi.org/10.1016/J.CYTO.2014.05.024
Wong CE, Paratore C, Dours-Zimmermann MT et al (2006) Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol 175:1005–1015. https://doi.org/10.1083/JCB.200606062
Wood NR, Garritson J, Mathias A et al (2022) Moderate intensity endurance and resistance exercise attenuates cachexia in tumor-bearing mice. Anticancer Res 42:397–405. https://doi.org/10.21873/ANTICANRES.15498
Wu H, Yu Y, Huang H et al (2020) Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 180:107-121.e17. https://doi.org/10.1016/J.CELL.2019.11.027
Wu MA, Lopez G, Nebuloni M et al (2021) Lung histopathologic clusters in severe COVID-19: a link between clinical picture and tissue damage. Crit Care. https://doi.org/10.1186/S13054-021-03846-5
Xie W, Strong JA, Zhang JM (2017) Active nerve regeneration with failed target reinnervation drives persistent neuropathic pain. eNeuro. https://doi.org/10.1523/ENEURO.0008-17.2017
Xu J, Ye Y, Zhang H et al (2016) Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine. https://doi.org/10.1097/MD.0000000000002502
Xu K, Wei Y, Giunta S et al (2021) Do inflammaging and coagul-aging play a role as conditions contributing to the co-occurrence of the severe hyper-inflammatory state and deadly coagulopathy during COVID-19 in older people? Exp Gerontol. https://doi.org/10.1016/J.EXGER.2021.111423
Yan H, Gao W, Pan Z et al (2012) The expression of α-SMA in the painful traumatic neuroma: potential role in the pathobiology of neuropathic pain. J Neurotrauma 29:2791–2797. https://doi.org/10.1089/NEU.2012.2502
Yaqoub L, Elgendy IY, Pepine CJ (2021) Sex and gender differences in COVID-19: more to be learned! Am Heart J Plus 3:100011. https://doi.org/10.1016/J.AHJO.2021.100011
Yeh CF, Li WY, Chu PY et al (2016) Pretreatment pain predicts perineural invasion in oral squamous cell carcinoma: a prospective study. Oral Oncol 61:115–119. https://doi.org/10.1016/J.ORALONCOLOGY.2016.07.016
Yehia R, Schaalan M, Abdallah DM et al (2021) Impact of TNF-α gene polymorphisms on pancreatic and non-small cell lung cancer-induced cachexia in adult Egyptian patients: a focus on pathogenic trajectories. Front Oncol. https://doi.org/10.3389/FONC.2021.783231
Yi M, Zheng X, Niu M et al (2022) Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. https://doi.org/10.1186/S12943-021-01489-2
Yip RML, Yim CW (2021) Role of interleukin 6 inhibitors in the management of rheumatoid arthritis. J Clin Rheumatol 27:E516–E524. https://doi.org/10.1097/RHU.0000000000001293
Zhang L, Zhao Y, Lu Y et al (2020a) Effects of vacuum sealing drainage to improve the therapeutic effect in patients with orthopedic trauma and to reduce post-operative infection and lower-limb deep venous thrombosis. Exp Ther Med. https://doi.org/10.3892/ETM.2020.8941
Zhang T, Wang XF, Wang ZC et al (2020b) Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. https://doi.org/10.1016/J.BIOPHA.2020.110287
Zhang R, Roque DM, Reader J, Lin J (2022) Combined inhibition of IL-6 and IL-8 pathways suppresses ovarian cancer cell viability and migration and tumor growth. Int J Oncol. https://doi.org/10.3892/IJO.2022.5340
Zhou YQ, Liu Z, Liu ZH et al (2016) Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. https://doi.org/10.1186/S12974-016-0607-6
Ziegler CGK, Allon SJ, Nyquist SK et al (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016-1035.e19. https://doi.org/10.1016/J.CELL.2020.04.035
Zigmond RE, Echevarria FD (2019) Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol 173:102–121. https://doi.org/10.1016/J.PNEUROBIO.2018.12.001
Živicová V, Lacina L, Mateu R et al (2017) Analysis of dermal fibroblasts isolated from neonatal and child cleft lip and adult skin: Developmental implications on reconstructive surgery. Int J Mol Med 40:1323–1334. https://doi.org/10.3892/IJMM.2017.3128
Zou X, Chen K, Zou J et al (2020) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14:185–192. https://doi.org/10.1007/S11684-020-0754-0
Acknowledgements
The authors are grateful to Šárka Takáčová for English style and grammar revision. The authors thank LPR Praha (League against Cancer Prague) for sustained support.
Funding
This research was funded by the Ministry of Education, Youth and Sports of the Czech Republic, project “Centre for Tumour Ecology – Research of the Cancer Microenvironment Supporting Cancer Growth and Spread,” No. CZ.02.1.01/0.0/0.0/16_019/0000785.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.S.J.; preparation, P.G., K.S.J., J.B., M.H., L.L., M.J., A.Š., K.S., H.H., A.R.; writing – review and editing, K.S.J., P.G., L.L., A.Š.; funding acquisition, K.S.J., A.Š. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
K.S.J., M.J. and J.B. are inventors of US patent no. 11,246,874 B1 and cooperate with Oxygen Biotech LLC 108 W 13th St. Wilmington DE 19801. This company had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript or in the decision to publish the results. The other authors, i.e. P.G., M.H., A.Š., L.L., A.R., H.H. and K.S., declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gál, P., Brábek, J., Holub, M. et al. Autoimmunity, cancer and COVID-19 abnormally activate wound healing pathways: critical role of inflammation. Histochem Cell Biol 158, 415–434 (2022). https://doi.org/10.1007/s00418-022-02140-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-022-02140-x