Abstract
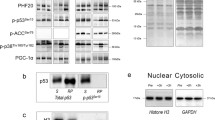
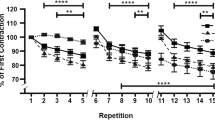
Early responses of stress-sensing proteins, muscle LIM protein (MLP), ankyrin repeat proteins (Ankrd1/CARP and Ankrd2/Arpp) and muscle-specific RING finger proteins (MuRF1 and MuRF2), along the titin molecule were investigated in the present experiment after submaximal exhaustive exercise. Ten healthy men performed continuous drop jumping unilaterally on a sledge apparatus with a submaximal height until complete exhaustion. Five stress-sensing proteins were analysed by mRNA measurements from biopsies obtained immediately and 3 h after the exercise from exercised vastus lateralis muscle while control biopsies were obtained from non-exercised legs before the exercise. Decreased maximal jump height and increased serum creatine kinase activities as indirect markers for muscle damage and HSP27 immunostainings on muscle biopsies as a direct marker for muscle damage indicated that the current exercised protocol caused muscle damage. mRNA levels for four (MLP, Ankrd1/CARP, MuRF1 and MuRF2) out of the five studied stress sensors significantly (p < 0.05) increased 3 h after fatiguing exercise. The magnitude of MLP and Ankrd2 responses was related to the proportion of type 1 myofibres. Our data showed that the submaximal exhaustive exercise with subject’s own physical fitness level activates titin-based stretch-sensing proteins. These results suggest that both degenerative and regenerative pathways are activated in very early phase after the exercise or probably already during the exercise. Activation of these proteins represents an initial step forward adaptive remodelling of the exercised muscle and may also be involved in the initiation of myofibre repair.


Similar content being viewed by others
References
Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, Thierse HJ, Witt CC, Linke A, Schuler G, Labeit S (2008) Induction of MuRF1 is essential for TNF-alpha-induced loss of muscle function in mice. J Mol Biol 384:48–59
Aihara Y, Kurabayashi M, Tanaka T, Takeda SI, Tomaru K, Sekiguchi KI, Ohyama Y, Nagai R (2000) Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/threonine kinase-dependent pathways. J Mol Cell Cardiol 32:1401–1414
Barash IA, Mathew L, Lahey M, Greaser ML, Lieber RL (2005) Muscle LIM protein plays both structural and functional roles in skeletal muscle. Am J Physiol Cell Physiol 289:C1312–C1320
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Carson JA, Nettleton D, Reecy JM (2002) Differential gene expression in the rat soleus muscle during early work overload-induced hypertrophy. FASEB J 16:207–209
Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S (2001) Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306:717–726
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81:S52–S69
Hentzen ER, Lahey M, Peters D, Mathew L, Barash IA, Fridén J, Lieber RL (2006) Stress-dependent and -independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. J Physiol 570:157–167
Jasnic-Savovic J, Krause S, Savic S, Kojic A, Kovcic V, Boskovic S, Nestorovic A, Rakicevic L, Schreiber-Katz O, Vogel JG, Schoser BG, Walter MC, Valle G, Radojkovic D, Faulkner G, Kojic S (2016) Differential expression and localization of Ankrd2 isoforms in human skeletal and cardiac muscles. Histochem Cell Biol 146:569–584
Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111:943–955
Koch AJ, Pereira R, Machado M (2014) The creatine kinase response to resistance exercise. J Musculoskelet Neuronal Interact 14:68–77
Kojic S, Radojkovic D, Faulkner G (2011) Muscle ankyrin repeat proteins: their role in striated muscle function in health and disease. Crit Rev Clin Lab Sci 48:269–294
Kostek MC, Chen YW, Cuthbertson DJ, Shi R, Fedele MJ, Esser KA, Rennie MJ (2007) Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol Genom 31:42–52
Kötter S, Unger A, Hamdani N, Lang P, Vorgerd M, Nagel-Steger L, Linke WA (2014) Human myocytes are protected from titin aggregation-induced stiffening by small heat shock proteins. J Cell Biol 204:187–202
Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H (2008) Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 376:1224–1236
Krüger M, Kötter S (2016) Titin, a central mediator for hypertrophic signaling, exercise-induced mechanosignaling and skeletal muscle remodeling. Front Physiol. doi:10.3389/fphys.2016.00076
Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edström L, Ehler E, Udd B, Gautel M (2005) The kinase domain of titin controls muscle gene expression and protein turnover. Science 308:1599–1603
Lange S, Ehler E, Gautel M (2006) From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol 16:11–18
Laure L, Suel L, Roudaut C, Bourg N, Ouali A, Bartoli M, Richard I, Danièle N (2009) Cardiac ankyrin repeat protein is a marker of skeletal muscle pathological remodelling. FEBS J 276:669–684
Lehti TM, Silvennoinen M, Kivelä R, Kainulainen H, Komulainen J (2007) Effects of streptozotocin-induced diabetes and physical training on gene expression of titin-based stretch-sensing complexes in mouse striated muscle. Am J Physiol Endocrinol Metab 292:E533–E542
Lehti M, Kivelä R, Komi P, Komulainen J, Kainulainen H, Kyröläinen H (2009) Effects of fatiguing jumping exercise on mRNA expression of titin-complex proteins and calpains. J Appl Physiol (1985) 106:1419–1424
Mayans O, Labeit S (2012) MuRFs: specialized members of the TRIM/RBCC family with roles in the regulation of the trophic state of muscle and its metabolism. Adv Exp Med Biol 770:119–129
Mckoy G, Hou Y, Yang SY, Vega Avelaira D, Degens H, Goldspink G, Coulton GR (2005) Expression of Ankrd2 in fast and slow muscles and its response to stretch are consistent with a role in slow muscle function. J Appl Physiol (1985) 98:2337–2343
Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S (2003) The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol 333:951–964
Moriscot AS, Baptista IL, Bogomolovas J, Witt C, Hirner S, Granzier H, Labeit S (2010) MuRF1 is a muscle fiber-type II associated factor and together with MuRF2 regulates type-II fiber trophicity and maintenance. J Struct Biol 170:344–353
Mymrikov EV, Seit-Nebi AS, Gusev NB (2011) Large potentials of small heat shock proteins. Physiol Rev 91:1123–1159
North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH (1999) A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet 21:353–354
Pallavicini A, Kojić S, Bean C, Vainzof M, Salamon M, Ievolella C, Bortoletto G, Pacchioni B, Zatz M, Lanfranchi G, Faulkner G, Valle G (2001) Characterization of human skeletal muscle Ankrd2. Biochem Biophys Res Commun 285:378–386
Paulsen G, Lauritzen F, Bayer ML, Kalhovde JM, Ugelstad I, Owe SG, Hallén J, Bergersen LH, Raastad T (2009) Subcellular movement and expression of HSP27, alphaB-crystallin, and HSP70 after two bouts of eccentric exercise in humans. J Appl Physiol (1985) 107:570–582
Paulsen G, Egner I, Raastad T, Reinholt F, Owe S, Lauritzen F, Brorson SH, Koskinen S (2013) Inflammatory markers CD11b, CD16, CD66b, CD68, myeloperoxidase and neutrophil elastase in eccentric exercised human skeletal muscles. Histochem Cell Biol 139:691–715
Roberts MD, Childs TE, Brown JD, Davis JW, Booth FW (2012) Early depression of Ankrd2 and Csrp3 mRNAs in the polyribosomal and whole tissue fractions in skeletal muscle with decreased voluntary running. J Appl Physiol (1985) 112:1291–1299
Sayers SP, Clarkson PM (2001) Force recovery after eccentric exercise in males and females. Eur J Appl Physiol 84:122–126
Schneider AG, Sultan KR, Pette D (1999) Muscle LIM protein: expressed in slow muscle and induced in fast muscle by enhanced contractile activity. Am J Physiol Cell Physiol 276:C900–C906
Seto JT, Lek M, Quinlan KG, Houweling PJ, Zheng XF, Garton F, MacArthur DG, Raftery JM, Garvey SM, Hauser MA, Yang N, Head SI, North KN (2011) Deficiency of α-actinin-3 is associated with increased susceptibility to contraction-induced damage and skeletal muscle remodeling. Hum Mol Genet 20:2914–2927
Tskhovrebova L, Trinick J (2003) Titin: properties and family relationships. Nat Rev Mol Cell Biol 4:679–689
Tsukamoto Y, Senda T, Nakano T, Nakada C, Hida T, Ishiguro N, Kondo G, Baba T, Sato K, Osaki M, Mori S, Ito H, Moriyama M (2002) Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab Invest 82:645–655
Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S (2008) Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J 27:350–360
Acknowledgements
The authors thank all the test personnel for their work in the data collection. We also would like to express our special thanks to Ms. Aila Ollikainen and Mr. Risto Puurtinen for their assistance in the blood and muscle analyses. This work was supported by a grant from the Scientific Advisory Board for Defence, Finland, Academy of Finland (Research Council for Health, Grant No. 137981) and Foundation for Physical Activity and Public Health LIKES, Finland. Sheila S. Gagnon is supported in part as a doctoral student by Western University’s Bone and Joint Institute and the Collaborative Training Program in Musculoskeletal Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koskinen, S.O.A., Kyröläinen, H., Flink, R. et al. Human skeletal muscle type 1 fibre distribution and response of stress-sensing proteins along the titin molecule after submaximal exhaustive exercise. Histochem Cell Biol 148, 545–555 (2017). https://doi.org/10.1007/s00418-017-1595-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-017-1595-z