Abstract
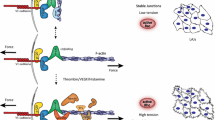
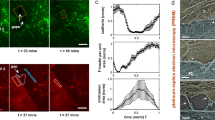
Force generation in non-muscle cells is vital for many cellular and tissue functions. Force-generating mechanisms include actomyosin-mediated contraction, actin polymerization that drives plasma membrane protrusions and filopodia as well as kinesin- and dynein-controlled transport of vesicles and organelles along the microtubule cytoskeleton. The actomyosin-mediated contractility and actin remodeling in both epithelium and endothelium were shown to have significant impact on cell migration, shape change and formation and control of intercellular junctions. In endothelium, contraction is supposed to control permeability for fluid and solutes. However, recent studies demonstrated the constitutive appearance of junction-associated intermittent lamellipodia (JAIL) that drive vascular endothelial cadherin (VE-cadherin) dynamics and control endothelial permeability. Since thrombin blocks JAIL formation and thus increases endothelial permeability, the concept of a simple Rho GTPase-controlled contraction, which is supposed to open endothelial junctions, becomes challenged. Furthermore, specific tyrosine phosphorylation sites of VE-cadherin and catenins have been shown to be involved in control of VE-cadherin-mediated cell adhesion. How the causal–mechanistic interdependency between contractility, VE-cadherin and catenin phosphorylation and JAIL-mediated dynamic remodeling of VE-cadherin is regulated is still an open question and needs to be further addressed.


Similar content being viewed by others
Notes
Cited from Dallos, P. and B. Fakler (2002). Prestin, a new type of motor protein. Nat Rev Mol Cell Biol 3(2): 104–111.
References
Abe K, Takeichi M (2008) EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci USA 105(1):13–19
Abella JV, Galloni C, Pernier J, Barry DJ, Kjaer S, Carlier MF, Way M (2016) Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nat Cell Biol 18(1):76–86
Abu Taha A, Schnittler HJ (2014) Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions. Cell Adh Migr 8(2):125–135
Abu Taha A, Taha M, Seebach J, Schnittler HJ (2014) ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Mol Biol Cell 25(2):245–256
Achler C, Filmer D, Merte C, Drenckhahn D (1989) Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. J Cell Biol 109(1):179–189
Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE (2008) Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol 294(3):H1188–H1196
Baumer Y, Spindler V, Werthmann RC, Bunemann M, Waschke J (2009) Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol 220(3):716–726
Braak E, Drenckhahn D, Unsicker K, Groschel-Stewart U, Dahl D (1978) Distribution of myosin and the glial fibrillary acidic protein (GFA protein) in rat spinal cord and in the human frontal cortex as revealed by immunofluorescence microscopy. Cell Tissue Res 191(3):493–499
Breslin JW, Zhang XE, Worthylake RA, Souza-Smith FM (2015) Involvement of local lamellipodia in endothelial barrier function. PLoS ONE 10(2):e0117970
Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR (2014) Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346(6209):1254211
Cudmore S, Cossart P, Griffiths G, Way M (1995) Actin-based motility of vaccinia virus. Nature 378(6557):636–638
Dallos P, Fakler B (2002) Prestin, a new type of motor protein. Nat Rev Mol Cell Biol 3(2):104–111
Drenckhahn D (1983) Cell motility and cytoplasmic filaments in vascular endothelium. In: Messmer K, Hammersen F (eds) Structure and function of endothelial cells, 1st Bodensee symposium on microcirculation, Lindau, October 1982, vol 1. Karger, Basel, pp 53–70
Drenckhahn D, Franke RP (1988) Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59(5):673–682
Drenckhahn D, Groschel-Stewart U (1977) Localization of myosin and actin in ocular nonmuscle cells. Immunofluorescence-microscopic, biochemical, and electron-microscopic studies. Cell Tissue Res 181(4):493–503
Drenckhahn D, Groschel-Stewart U (1978) Immunohistochemical localization of myosin and actin in various nonmuscle cell types of rat eyes. Verh Anat Ges 72:279–282
Drenckhahn D, Wagner J (1986) Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol 102(5):1738–1747
Drenckhahn D, Groschel-Stewart U, Unsicker K (1977) Immunofluorescence-microscopic demonstration of myosin and actin in salivary glands and exocrine pancreas of the rat. Cell Tissue Res 183(2):273–279
Esser S, Lampugnani MG, Corada M, Dejana E, Risau W (1998) Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111(Pt 13):1853–1865
Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M (1998) Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 273(34):21867–21874
Franke RP, Grafe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D (1984) Induction of human vascular endothelial stress fibres by fluid shear stress. Nature 307(5952):648–649
He DZ, Lovas S, Ai Y, Li Y, Beisel KW (2014) Prestin at year 14: progress and prospect. Hear Res 311:25–35
Hofer D, Ness W, Drenckhahn D (1997) Sorting of actin isoforms in chicken auditory hair cells. J Cell Sci 110(Pt 6):765–770
Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J (2012) Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol 196(5):641–652
Ireton K, Rigano LA, Polle L, Schubert WD (2014) Molecular mechanism of protrusion formation during cell-to-cell spread of Listeria. Front Cell Infect Microbiol 4:21
Kemler R, Ozawa M (1989) Uvomorulin-catenin complex: cytoplasmic anchorage of a Ca2+-dependent cell adhesion molecule. BioEssays 11(4):88–91
Koob R, Zimmermann M, Schoner W, Drenckhahn D (1988) Colocalization and coprecipitation of ankyrin and Na+, K+-ATPase in kidney epithelial cells. Eur J Cell Biol 45(2):230–237
Kronstein R, Seebach J, Grossklaus S, Minten C, Engelhardt B, Drab M, Liebner S, Arsenijevic Y, Taha AA, Afanasieva T, Schnittler HJ (2012) Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc Res 93(1):130–140
Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E (1992) A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol 118(6):1511–1522
Martorell L, Martinez-Gonzalez J, Rodriguez C, Gentile M, Calvayrac O, Badimon L (2008) Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost 99(2):305–315
Mooseker MS (1976) Brush border motility. Microvillar contraction in triton-treated brush borders isolated from intestinal epithelium. J Cell Biol 71(2):417–433
Mooseker MS, Tilney LG (1975) Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol 67(3):725–743
Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E (2012) Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 3:1208
Ruf W (2003) PAR1 signaling: more good than harm? Nat Med 9(3):258–260
Schnittler HJ, Wilke A, Gress T, Suttorp N, Drenckhahn D (1990) Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J Physiol 431:379–401
Schnittler H, Taha M, Schnittler MO, Taha AA, Lindemann N, Seebach J (2014) Actin filament dynamics and endothelial cell junctions: the Ying and Yang between stabilization and motion. Cell Tissue Res 355(3):528–543
Seebach J, Madler HJ, Wojciak-Stothard B, Schnittler HJ (2005) Tyrosine phosphorylation and the small GTPase rac cross-talk in regulation of endothelial barrier function. Thromb Haemost 94(3):620–629
Seebach J, Donnert G, Kronstein R, Werth S, Wojciak-Stothard B, Falzarano D, Mrowietz C, Hell SW, Schnittler HJ (2007) Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Cardiovasc Res 75(3):596–607
Seebach J, Taha AA, Lenk J, Lindemann N, Jiang X, Brinkmann K, Bogdan S, Schnittler HJ (2015) The Cell BorderTracker, a novel tool to quantitatively analyze spatiotemporal endothelial junction dynamics at the subcellular level. Histochem Cell Biol 144(6):517–532
Seebach J, Cao J, Schnittler H (2016) Quantitative dynamics of VE-cadherin at endothelial cell junctions at a glance: basic requirements and current concepts. Discoveries 4(3), e63
Suzuki S, Sano K, Tanihara H (1991) Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul 2(4):261–270
Takeichi M (1990) Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem 59:237–252
Trani M, Dejana E (2015) New insights in the control of vascular permeability: vascular endothelial-cadherin and other players. Curr Opin Hematol 22(3):267–272
Unsicker K, Drenckhahn D, Gruschel-Stewart U (1978) Further immunofluorescence-microscopic evidence for myosin in various peripheral nerves. Cell Tissue Res 188(2):341–344
van Nieuw Amerongen GP, Musters RJ, Eringa EC, Sipkema P, van Hinsbergh VW (2008) Thrombin-induced endothelial barrier disruption in intact microvessels: role of RhoA/Rho kinase-myosin phosphatase axis. Am J Physiol Cell Physiol 294(5):C1234–C1241
Vestweber D (2015) How leukocytes cross the vascular endothelium. Nat Rev Immunol 15(11):692–704
Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D (2014) Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol 15(3):223–230
Wozniak MA, Chen CS (2009) Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10(1):34–43
Acknowledgments
This work was supported by grants from the German Research Council to H.S. (DFG INST 2105/24–1 and SCHN 430/6–2), the Excellence Cluster Cells In Motion (CIM), and WWU-Münster flexible fund to H.S (FF-2014-15).
Author information
Authors and Affiliations
Corresponding author
Additional information
This short overview is dedicated to D. Drenckhahn.
Rights and permissions
About this article
Cite this article
Schnittler, H. Contraction of endothelial cells: 40 years of research, but the debate still lives. Histochem Cell Biol 146, 651–656 (2016). https://doi.org/10.1007/s00418-016-1501-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1501-0