Abstract
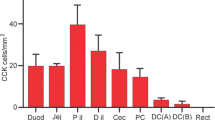
Information concerning the cellular localization of cholecystokinin (CCK)-1 receptors has been discrepant and remained scanty at ultrastructural levels. The present immunohistochemical study at light and electron microscopic levels revealed the distinct localization of CCK1 receptors in visceral organs. Immunohistochemistry by use of a purified antibody against mouse CCK1 receptor was applied to fixed tissue sections of the pancreas, gallbladder, stomach, and intestine of mice. A silver-intensified immunogold method revealed the subcellular localization under electron microscope. The immunoreactivity for CCK1 receptors was selectively found in the basolateral membrane of pancreatic acinar cells and gastric chief cells but was absent in pancreatic islets and gastric D cells. Another intense expression in the gut was seen in the myenteric nerve plexus of the antro-duodenal region and some populations of c-Kit-expressing pacemaker cells in the duodenal musculature. The gallbladder contained smooth muscle fibers with an intense immunoreactivity of CCK1 receptors on cell surfaces. The restricted localization of CCK1 receptors on the basolateral membrane of pancreatic acinar cells and gastric chief cells, along with their absence in the islets of Langerhans and gastric D cells, provides definitive information concerning the regulatory mechanism by circulating CCK. Especially, the subcellular localization in the acinar cells completes the investigation for the detection of circulating CCK by the basolateral membrane.





Similar content being viewed by others
References
Blandizzi C, Lazzeri G, Colucci R, Carignani D, Tognetti M, Baschiera F, Tacca MD (1999) CCK1 and CCK2 receptors regulate gastric pepsinogen secretion. Eur J Pharmacol 373:71–84
Bolender RP (1974) Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol 61:269–287
Bourassa J, Lainé J, Kruse M-L, Gagnon M-C, Calvo É, Morisset J (1999) Ontogeny and species differences in the pancreatic expression and localization of the CCKA receptors. Biochem Biophys Res Commun 260:820–828
Buchan AMJ, Meloche RM, Kwok YN, Kofod H (1993) Effect of cholecystokinin and secretin on somatostatin release from cultured antral cells. Gastroenterology 104:1414–1419
Criddle DN, Booth DM, Mukherjee R, McLaughlin E, Green GM, Sutton R, Petersen OH, Reeve JR Jr (2009) Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 297:G1085–G1092
de Weerth A, Pisegna JR, Huppi K, Wank SA (1993) Molecular cloning, functional expression and chromosomal localization of the human cholecystokinin type A receptor. Biochem Biophys Res Commun 194:811–818
Glatzle J, Kreis ME, Kawano K, Raybould HE, Zittel TT (2001) Postprandial neuronal activation in the nucleus of the solitary tract is partly mediated by CCK-A receptors. Am J Physiol Regul Integr Comp Physiol 281:R222–R229
Habara Y, Kanno T (1991) Dose-dependency in spatial dynamics of [Ca2+]c in pancreatic acinar cells. Cell Calcium 12:533–542
Helander HF, Wong H, Poorkhalkali N, Walsh JH (1997) Immunohistochemical localization of gastrin/CCK-B receptors in the dog and guinea-pig stomach. Acta Physiol Scand 159:313–320
Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD (2001) Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology 121:1380–1390
Kageyama H, Kita T, Horie S, Takenoya F, Funahashi H, Kato S, Hirayama M, Lee EY, Sakurai J, Inoue S, Shioda S (2005) Immunohistochemical analysis of cholecystokinin A receptor distribution in the rat pancreas. Regul Pept 126:137–143
Karlsson S, Sundler F, Ahrén B (1998) CCK receptor subtype in insulin-producing cells: a combined functional and in situ hybridization study in rat islets and a rat insulinoma cell line. Regul Pept 78:95–1103
Ladenheim EE, Speth RC, Ritter RC (1988) Reduction of CCK-8 binding in the nucleus of the solitary tract in unilaterally nodosectomized rats. Brain Res 474:125–129
Li Q, Luo X, Muallem S (2004) Functional mapping of Ca2+ signaling in plasma membrane microdomains of polarized cells. J Biol Chem 279:27837–27840
Lin CW, Bianchi BR, Miller TR, Witte DG, Wolfram CAW (1992) Both CCK-A and CCK-B/gastrin receptors mediate pepsinogen release in guinea pig gastric glands. Am J Physiol 262:G1113–G1120
Lloyd KC, Raybould HE, Walsh JH (1992a) Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am J Physiol 263:G287–G292
Lloyd KC, Maxwell V, Kovacs TO, Miller J, Walsh JH (1992b) Cholecystokinin receptor antagonist MK-329 blocks intestinal fat-induced inhibition of meal-stimulated gastric acid secretion. Gastroenterology 102:131–138
Lloyd KCK, Maxwell V, Chuang C-N, Wong HC, Soll AH, Walsh JH (1994) Somatostatin is released in response to cholecystokinin by activation of type A CCK receptors. Peptides 15:223–227
Mantyh CR, Pappas TN, Vigna SR (1994) Localization of cholecystokinin A and cholecystokinin B/gastrin receptors in the canine upper gastrointestinal tract. Gastroenterology 107:1019–1030
Mawe GM (1991) The role of cholecystokinin in ganglionic transmission in the guinea-pig gall-bladder. J Physiol 439:89–102
Mizushima S, Nagata S (1990) pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res 18:5322
Moran TH, Norgren R, Crosby RJ, McHugh PR (1990) Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 526:95–102
Morgan KG, Schmalz PF, Go VLW, Szurszewski JH (1978) Electrical and mechanical effects of molecular variants of CCK on antral smooth muscle. Am J Physiol 235:E324–E329
Morisset J, Julien S, Lainé J (2003) Localization of cholecystokinin receptor subtypes in the endocrine pancreas. J Histochem Cytochem 51:1501–1513
Murphy RB, Smith GP, Gibbs J (1987) Pharmacological examination of cholecystokinin (CCK-8)-induced contractile activity in the rat isolated pylorus. Peptides 8:127–134
Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MG, Ghaneh P, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Green GM, Reeve JR Jr, Petersen OH, Sutton R (2008) Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 135:632–641
Ohlsson B, Borg K, Mulder H, Rehfeld JF, Axelson J, Sundler F (2000) Continuous infusion of cholecystokinin leads to down-regulation of the cholecystokinin-A receptor in the rat pancreas. Scand J Gastroenterol 35:612–618
Owyang C, Logsdon CD (2004) New insights into neurohormonal regulations of pancreatic secretion. Gastroenterology 127:957–969
Patterson LM, Zheng H, Ward SM, Berthoud H-R (2001) Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res 305:11–23
Raybould HE, Gayton RJ, Dockray GJ (1988) Mechanisms of action of peripherally administrated cholecystokinin octapeptide on brain stem neurons in the rat. J Neurosci 8:3018–3024
Reubi JA, Waser B, Läderach U, Stettler C, Friess H, Halter F, Schmassmann A (1997) Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology 112:1197–1205
Rozengurt E, Walsh JH (2001) Gastrin, CCK, sinaling, and cancer. Annu Rev Physiol 63:49–76
Scheurer U, Varga L, Drack E, Bürki H-R, Halter F (1983) Measurement of cholecystokinin octapeptide-induced motility of rat, pylorus, and duodenum in vitro. Am J Physiol 244:G261–G265
Schjoldager B, Molero X, Miller LJ (1989) Functional and biochemical characterization of the human gallbladder muscularis cholecystokinin receptor. Gastroenterology 96:1119–1125
Schmidt WE, Schenk S, Nustede R, Holst JJ, Fölsch UR, Creutzfeldt W (1994) Cholecystokinin is a negative regulator of gastric acid secretion and postprandial release of gastrin in humans. Gastroenterology 107:1610–1620
Schmitz F, Göke MN, Otte J-M, Schrader H, Reimann B, Kruse M-L, Siegel EG, Peters J, Herzig K-H, Fölsch U, Schmidt WE (2001) Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul Pept 102:101–110
Schulz S, Röcken C, Mawrin C, Schulz S (2005) Immunohistochemical localization of CCK1 cholecystokinin receptors in normal and neoplastic human tissues. J Clin Endocrinol Metab 90:6149–6155
Schweiger M, Erhard MH, Amselgruber WM (2000) Cell-specific localization of the cholecystokininA receptor in the porcine pancreas. Anat Histol Embryol 29:357–361
Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, Muallem S (2001) Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem 276:44146–44156
Singer MV, Niebergall-Roth E (2009) Secretion from acinar cells of the exocrine pancreas: role of enteropancreatic reflexes and cholecystokinin. Cell Biol Int 33:1–9
Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ (1981) Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213:1036–1037
Smith GT, Moran TH, Coyle JT, Kuhar MJ, O’Donahue TL, McHugh PR (1984) Anatomic localization of cholecystokinin receptors to the pyloric sphincter. Am J Physiol 246:R127–R130
Smith GP, Jerome C, Norgren R (1989) Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol 249:R638–R641
Sternini C, Wong H, Pham T, de Giorgio R, Miller LJ, Kuntz SM, Reeve JR Jr, Walsh JH, Raybould HE (1999) Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology 117:1136–1146
Suzuki S, Takiguchi S, Sato N, Kanai S, Kawanami T, Yoshida Y, Miyasaka K, Takata Y, Funakoshi A, Noda T (2001) Importance of CCK-A receptor for gallbladder contraction and pancreatic secretion: a study in CCK-A receptor knockout mice. Jpn J Physiol 51:585–590
Tang LH, Miller MD, Goldenring JR, Modlin IM, Hersey SJ (1993) Partial agonism by gastrin for a cholecystokinin receptor mediating pepsinogen secretion. Am J Physiol 265:G865–G872
Tang C, Biemond I, Lamers CBHW (1996) Cholecystokinin receptors in human pancreas and gallbladder muscle: a comparative study. Gastroenterology 111:1621–1626
Walsh JH (1987) Gastrointestinal hormones. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 2nd edn. Raven Press, New York, pp 181–253
Wank SA (1995) Cholecystokinin receptors. Am J Physiol 269:G628–G646
Williams JA (1982) Cholecystokinin: a hormone and a neurotransmitter. Biomed Res 3:107–115
Yamasaki M, Matsui M, Watanabe M (2010) Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci 30:4408–4418
Acknowledgments
This study is supported by Grants-in-Aid for Scientific Research 19100005 (to M. W.) and Core Research for Evolutional Science and Technology (CREST) from Japan Science and Technology Cooperation (to M.W.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

418_2014_1281_MOESM1_ESM.jpg
Immunostaining of the CCK1 receptor in wild-type (A, C, and E) and CCK1 receptor-deficient mice (B, D, and F). The immunoreactivities in the muscle layer of the gallbladder (A), the chief cells of the stomach (C), and ICC cells of the duodenum disappear in CCK1 receptor-deficient mice (B, D, and F). Nuclei are stained green in A, B, E, and F. Bar, 20 μm (A, B, E, and F), 50 μm (C and D) (JPEG 786 kb)

418_2014_1281_MOESM2_ESM.jpg
Immunostaining of Gαq11 in the pancreas (A), stomach (B), and gallbladder (C). The basolateral membrane of pancreatic acinar cells and gastric chief cells is positive in reaction. In figure B, the positive immunoreactivities are also found in endocrine cells (arrow) and lamina muscularis mucosae. The gallbladder (C) displays the smooth muscle layer with a Gαq11 immunoreactivity. Nuclei are counterstained green with SYTOX green. Bar, 20 μm (JPEG 479 kb)

418_2014_1281_MOESM3_ESM.jpg
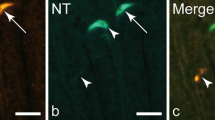
Immunostaining of the CCK1 receptor in the nodose ganglion (A and B) and the myenteric nerve plexus of the duodenum (C). Several neurons in the nodose ganglion show a positive immunoreactivity for the CCK1 receptor. Nuclei are stained green in figure B. In figure C, double staining with PGP9.5, a neuronal marker, of a whole mount preparation demonstrates a dot-like distribution of the CCK1 receptor immunoreactivity on the myenteric nerve plexus. Arrows indicate immunoreactive cell bodies of neurons. In figure D, in situ hybridization analysis for the CCK1 receptor and neuron-specific enolase (NSE) on a tissue section detects a CCK1 receptor mRNA-expressing neuron in the myenteric nerve plexus of the duodenum. Bar, 50 μm (A), 20 μm (B–D) (JPEG 553 kb)

418_2014_1281_MOESM4_ESM.jpg
CCK1 receptor immunoreactivities in the pancreas of the guinea pig (A) and rat (B). The CCK1 receptor is localized in pancreatic acinar cells but not islets (I). In figure A, nuclei are stained green, while in figure B insulin-containing cells are stained green. Bar, 20 μm (JPEG 519 kb)

418_2014_1281_MOESM5_ESM.jpg
Surface view of pancreatic acinar cells by scanning electron microscope (SEM). After removal of the basement membrane and connective tissue by the NaOH maceration method, the basal (B) and lateral (L) surfaces of acinar cells were observed by SEM. The basal and lateral surfaces are equipped with small domains composed of microfolds (arrows). An asterisk indicates the lumen of an acinus. Bar, 1 μm (JPEG 463 kb)
Rights and permissions
About this article
Cite this article
Konno, K., Takahashi-Iwanaga, H., Uchigashima, M. et al. Cellular and subcellular localization of cholecystokinin (CCK)-1 receptors in the pancreas, gallbladder, and stomach of mice. Histochem Cell Biol 143, 301–312 (2015). https://doi.org/10.1007/s00418-014-1281-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1281-3