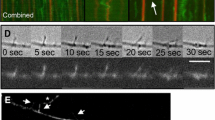
Abstract
The neuronal growth cone plays a crucial role in the development of the nervous system. This highly motile structure leads the axon to its final destination by translating guidance cues into cytoskeletal rearrangements. Recently, vascular endothelial growth factor (VEGF), which is essential for angiogenesis and vascular sprouting, has been found to exert a trophic activity also on neurons, leading to an increased axonal outgrowth, similar to the well-known nerve growth factor (NGF). The neurotrophic properties of VEGF are likely to be promoted via the VEGF receptor 2 (VEGFR-2) and neuropilin-1 (NRP-1). In the long term, VEGF attracts and influences the growth cone velocity and leads to growth cone enlargement. The present study focuses on immediate VEGF effects using RFP-actin and GFP-NF-M microinjected chicken dorsal root ganglia for live cell imaging of the neuronal growth cone. We analyzed actin and neurofilament dynamics following VEGF and NGF treatment and compared the effects. Furthermore, key signaling pathways of VEGF were investigated by specific blocking of VEGFR-2 or NRP-1. With the aid of confocal laser scanning microscopy and stimulated emission depletion microscopy, we show for the first time that VEGF has a quick effect on the actin-cytoskeleton, since actin rearrangements were identifiable within a few minutes, leading to a dramatically increased motion. Moreover, these effects were strongly enhanced by adding both VEGF and NGF. Most notably, the effects were inhibited by blocking VEGFR-2, therefore we propose that the immediate effects of VEGF on the actin-cytoskeleton are mediated through VEGFR-2.







Similar content being viewed by others
References
Argiro V, Bunge MB, Johnson MI (1984) Correlation between growth form and movement and their dependence on neuronal age. J Neurosci 4(12):3051–3062
Bentley D, Toroian-Raymond A (1986) Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature 323(6090):712–715
Böcker-Meffert S, Rosenstiel P, Röhl C, Warneke N, Held-Feindt J, Sievers J, Lucius R (2002) Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci 43(6):2021–2026
Brockington A, Lewis C, Wharton S, Shaw PJ (2004) Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol 30(5):427–446
Brown JA, Bridgman PC (2009) Disruption of the cytoskeleton during Semaphorin 3A induced growth cone collapse correlates with differences in actin organization and associated binding proteins. Dev Neurobiol 69(10):633–646
Brown JA, Wysolmerski RB, Bridgman PC (2009) Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell 20(4):1167–1179
Cajal SRY (1890) À quelle époque apparaissent les expansions des cellules nerveuses de la moëlle épinière du poulet? Anat Anz 21–22:609–639
Carmeliet P (2003) Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet 4(9):710–720
Cheng L, Jia H, Löhr M, Bagherzadeh A, Holmes DI, Selwood D, Zachary I (2004) Anti-chemorepulsive effects of vascular endothelial growth factor and placental growth factor-2 in dorsal root ganglion neurons are mediated via neuropilin-1 and cyclooxygenase-derived prostanoid production. J Biol Chem 279(29):30654–30661
Chien CB, Rosenthal DE, Harris WA, Holt CE (1993) Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron 11(2):237–251
Cohen S, Levi-Montalcini R, Hamburger V (1954) A nerve growth-stimulating factor isolated from Sarcom as 37 and 180. Proc Natl Acad Sci USA 40(10):1014–1018
Crawford Y, Ferrara N (2009) Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci 30(12):624–630
Dent EW, Gertler FB (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40(2):209–227
Dent EW, Kalil K (2001) Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci 21(24):9757–9769
Dent EW, Barnes AM, Tang F, Kalil K (2004) Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci 24(12):3002–3012
Dent EW, Gupton SL, Gertler FB (2011) The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 3(3)
Dickson BJ (2001) Rho GTPases in growth cone guidance. Curr Opin Neurobiol 11(1):103–110
Dickson BJ (2002) Molecular mechanisms of axon guidance. Science 298(5600):1959–1964
Evans AR, Euteneuer S, Chavez E, Mullen LM, Hui EE, Bhatia SN, Ryan AF (2007) Laminin and fibronectin modulate inner ear spiral ganglion neurite outgrowth in an in vitro alternate choice assay. Dev Neurobiol 67(13):1721–1730
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611
Ferrara N (2010) Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev 21(1):21–26
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18(1):4–25
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676
Foehring D, Brand-Saberi B, Theiss C (2012) VEGF-induced growth cone enhancement is diminished by inhibiting tyrosine-residue 1214 of VEGFR-2. Cells Tissues Organs Mar 20 (Epub ahead of print)
Forstreuter F, Lucius R, Mentlein R (2002) Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol 132(1–2):93–98
Fuh G, Garcia KC, de Vos AM (2000) The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem 275(35):26690–26695
Goldberg DJ, Burmeister DW (1986) Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol 103(5):1921–1931
He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90(4):739–751
Huber AB, Kolodkin AL, Ginty DD, Cloutier JF (2003) Signaling at the growth cone: ligand–receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci 26:509–563
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342
Jin KL, Mao XO, Greenberg DA (2000) Vascular endothelial growth factor rescues HN33 neural cells from death induced by serum withdrawal. J Mol Neurosci 14(3):197–203
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99(18):11946–11950
Jin K, Mao XO, Greenberg DA (2006) Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol 66(3):236–242
Kaufmann N, Wills ZP, Van Vactor D (1998) Drosophila Rac1 controls motor axon guidance. Development 125(3):453–461
Kelly RJ, Rixe O (2009) Axitinib—a selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target Oncol 4(4):297–305
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358(19):2039–2049
Kermer P, Klöcker N, Labes M, Bähr M (2000) Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci 20(2):2–8
Khaibullina AA, Rosenstein JM, Krum JM (2004) Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res 148(1):59–68
Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H (1997) Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19(5):995–1005
Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD (1997) Neuropilin is a semaphorin III receptor. Cell 90(4):753–762
Kovács Z, Ikezaki K, Samoto K, Inamura T, Fukui M (1996) VEGF and flt. Expression time kinetics in rat brain infarct. Stroke 27(10):1865–1872
Krum JM, Rosenstein JM (1998) VEGF mRNA and its receptor flt-1 are expressed in reactive astrocytes following neural grafting and tumor cell implantation in the adult CNS. Exp Neurol 154(1):57–65
Lafont F, Rouget M, Rousselet A, Valenza C, Prochiantz A (1993) Specific responses of axons and dendrites to cytoskeleton perturbations: an in vitro study. J Cell Sci 104(Pt 2):433–443
Lamalice L, Houle F, Jourdan G, Huot J (2004) Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 23(2):434–445
Levi-Montalcini R, Angeletti Pu (1963) Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol 7:653–659
Lowery LA, Van Vactor D (2009) The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 10(5):332–343
Mallavarapu A, Mitchison T (1999) Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol 146(5):1097–1106
Marín O, Valiente M, Ge X, Tsai LH (2010) Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol 2(2):a001834
Marko SB, Damon DH (2008) VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol 294(6):H2646–H2652
Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL (2005) Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 121(1):127–139
Marsh L, Letourneau PC (1984) Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol 99(6):2041–2047
Marsick BM, Flynn KC, Santiago-Medina M, Bamburg JR, Letourneau PC (2010) Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev Neurobiol 70(8):565–588
Marti HH, Risau W (1999) Angiogenesis in ischemic disease. Thromb Haemost 82(Suppl 1):44–52
Maskery S, Shinbrot T (2005) Deterministic and stochastic elements of axonal guidance. Annu Rev Biomed Eng 7:187–221
Matsumoto T, Claesson-Welsh L (2001) VEGF receptor signal transduction. Sci STKE 112:re21
Matsumoto T, Mugishima H (2006) Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis. J Atheroscler Thromb 13(3):130–135
Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M (2001) Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J 15(7):1218–1220
Meller K (1974) The reaggregation of neurons and their satellite cells in cultures of trypsin-dissociated spinal ganglia. Cell Tissue Res 152(2):175–183
Meller K (1992) Axoplasmic transport of horseradish peroxidase in single neurons of the dorsal root ganglion studied in vitro by microinjection. Cell Tissue Res 270(1):139–148
Meller K (1994) Cryo-electron microscopy of myelin treated with detergents. Cell Tissue Res 276(3):551–558
Meyer G, Feldman EL (2002) Signaling mechanisms that regulate actin-based motility processes in the nervous system. J Neurochem 83(3):490–503
Mitchison T, Kirschner M (1988) Cytoskeletal dynamics and nerve growth. Neuron 1(9):761–772
Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y (2002) The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc Med 12(1):13–19
Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR (2002) Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem 277(13):11410–11415
Oh H, Takagi H, Otani A, Koyama S, Kemmochi S, Uemura A, Honda Y (2002) Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): a mechanism contributing to VEGF-induced angiogenesis. Proc Natl Acad Sci USA 99(1):383–388
Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11(1):53–67
Park HT, Wu J, Rao Y (2002) Molecular control of neuronal migration. BioEssays 24(9):821–827
Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57(20):4593–4599
Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R (2008) Lifeact: a versatile marker to visualize F-actin. Nat Methods 5(7):605–607
Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4(6):446–456
Rosenstein JM, Mani N, Khaibullina A, Krum JM (2003) Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci 23(35):11036–11044
Rousseau S, Houle F, Landry J, Huot J (1997) p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15(18):2169–2177
Rousseau S, Houle F, Hout J (2000a) Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc Med 10:321–327
Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J (2000b) Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem 275:10661–10672
Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, Van Geyte K, Dhondt J, Dresselaers T, Renaud J, Aragones J, Zacchigna S, Geudens I, Gall D, Stroobants S, Mutin M, Dassonville K, Storkebaum E, Jordan BF, Eriksson U, Moons L, D’Hooge R, Haigh JJ, Belin MF, Schiffmann S, Van Hecke P, Gallez B, Vinckier S, Chédotal A, Honnorat J, Thomasset N, Carmeliet P, Meissirel C (2010) Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci 30(45):15052–15066
Schaefer AW, Kabir N, Forscher P (2002) Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol 158(1):139–152
Schratzberger P, Schratzberger G, Silver M, Curry C, Kearney M, Magner M, Alroy J, Adelman LS, Weinberg DH, Ropper AH, Isner JM (2000) Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med 6(4):405–413
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219(4587):983–985
Shibuya M (2008) Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 41(4):278–286
Silverman WF, Krum JM, Mani N, Rosenstein JM (1999) Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience 90(4):1529–1541
Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24:1217–1281
Soker S, Fidder H, Neufeld G, Klagsbrun M (1996) Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem 271(10):5761–5767
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92(6):735–745
Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M (2002) VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem 85(2):357–368
Sondell M, Kanje M (2001) Postnatal expression of VEGF and its receptor flk-1 in peripheral ganglia. NeuroReport 12(1):105–108
Sondell M, Lundborg G, Kanje M (1999) Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 19(14):5731–5740
Sondell M, Sundler F, Kanje M (2000) Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci 12(12):4243–4254
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111(12):1843–1851
Suter DM, Forscher P (2000) Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J Neurobiol 44(2):97–113
Suter DM, Schaefer AW, Forscher P (2004) Microtubule dynamics are necessary for SRC family kinase-dependent growth cone steering. Curr Biol 14(13):1194–1199
Symons MH, Mitchison TJ (1991) Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol 114(3):503–513
Theiss C, Napirei M, Meller K (2005) Impairment of anterograde and retrograde neurofilament transport after anti-kinesin antibody microinjection in chicken dorsal root ganglia. Eur J Cell Biol 84(1):29–43
Theiss C, Neuhaus A, Schliebs W, Erdmann R (2012) TubStain: a universal peptide-tool to label microtubules. Histochem Cell Biol (Epub ahead of print)
Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E (2006) Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem 281(18):12950–12958
Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C (2008) Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol 87:649–667
Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D (2003) Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J Biol Chem 278(49):48848–48860
Wettschureck N, Offermanns S (2002) Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med (Berl) 80(10):629–638
Whitaker GB, Limberg BJ, Rosenbaum JS (2001) Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J Biol Chem 276(27):25520–25531
Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB (2002) Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci 22(15):6401–6407
Wildanger D, Rittweger E, Kastrup L, Hell SW (2008) STED microscopy with a supercontinuum laser source. Opt Express 16:9614–9621
Wuestefeld R, Chen J, Meller K, Brand-Saberi B, Theiss C (2012) Impact of vegf on astrocytes: analysis of gap junctional intercellular communication, proliferation, and motility. Glia 60(6):936–947
Zachary I (2005) Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals 14(5):207–221
Zeng H, Dvorak HF, Mukhopadhyay D (2001) Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem 276(29):26969–26979
Zhu Y, Jin K, Mao XO, Greenberg DA (2003) Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J 17(2):186–193
Acknowledgments
The authors gratefully thank FoRUM (RUB) for financial support (F670-2009). Mrs L. Olbrich especially thanks the Heinrich und Alma Vogelsang-Stiftung for financial support in line with a graduation-scholarship. The authors further acknowledge T. Nguyen, B. Menzel and A. Lodwig for excellent technical assistance as well as A. Lenz for secretarial work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 1180 kb)
Supplementary material 2 (MPG 534 kb)
Rights and permissions
About this article
Cite this article
Olbrich, L., Foehring, D., Happel, P. et al. Fast rearrangement of the neuronal growth cone’s actin cytoskeleton following VEGF stimulation. Histochem Cell Biol 139, 431–445 (2013). https://doi.org/10.1007/s00418-012-1036-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-1036-y