Abstract
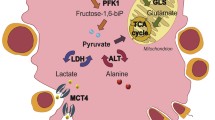
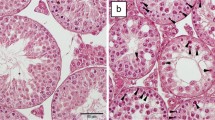
The present study examined the cellular localization of monocarboxylate transporters (MCTs), glucose transporters (GLUTs), and some glycolysis-related molecules in the murine female genital tract to demonstrate existence of lactate/pyruvate-dependent energy systems. MCT1, a major MCT subtype, was localized selectively in the ovarian granulosa, oviductal-ciliated cells, and vaginal epithelium; all localizations were associated with intense expressions of glycolytic enzymes. MCT1 was localized in the cell membrane of granulosa cells, including fine processes extending from cumulus cells toward oocytes. The cumulus cells and oocytes showed intense signals for lactate dehydrogenase (LDH)-A and -B, respectively. The basolateral membrane of oviductal-ciliated cells expressed MCT4 as well as MCT1, while adjacent non-ciliated cells contained an intense immunoreactivity for aldolase-C, a glycolytic enzyme. The expression of GLUTs in the ovary was generally weak with an intense expression of GLUT1 only in some vascular endothelia. The oviductal epithelium expressed GLUT1 and GLUT3, respectively, in the basolateral and apical membrane of non-ciliated cells. In the vagina, the basal layers of epithelium were immunolabeled for MCT1 with the entire length of cell membrane, and expressed abundantly both GLUT1 and LDH-A. The findings correspond well with the rich existence of lactate in the genital fluids and strongly suggest the active transport of lactate/pyruvate in the female reproductive tract, which provides favorable conditions for oocytes, sperms, and zygotes.




Similar content being viewed by others
References
Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S (2001) Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379
Biggers JD, Whittingham DG, Donahue RP (1967) The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA 58:560–567
Boris S, Barbes C (2000) Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2:543–546
Brauchi S, Rauch MC, Alfaro IE, Cea C, Concha II, Benos DJ, Reyes JG (2005) Kinetics, molecular basis, and differentiation of L-lactate transport in spermatogenic cells. Am J Physiol Cell Physiol 288:C523–C534
Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587:5591–5600
Cetica PD, Pintos LN, Dalvit GC, Beconi MT (1999) Effect of lactate dehydrogenase activity and isoenzyme localization in bovine oocytes and utilization of oxidative substrates on in vitro maturation. Theriogenology 51:541–550
Coonrod S, Vitale A, Duan C, Bristol-Gould S, Herr J, Goldberg E (2006) Testis-specific lactate dehydrogenase (LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J Androl 27:502–509
Donahue RP, Stern S (1968) Follicular cell support of oocyte maturation: production of pyruvate in vitro. J Reprod Fertile 17:395–398
Eppig JJ (1976) Analysis of mouse oogenesis in vitro. Oocyte isolation and the utilization of exogenous energy sources by growing oocytes. J Exp Zool 198:375–386
Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK (1989) Prevalence of hydrogen peroxidase-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 27:251–256
Feron O (2009) Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 92:329–333
Garcia CK, Goldstein JL, Pathak RK, Anderson RGW, Brown MS (1994) Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76:865–873
Garcia CK, Brown MS, Pathak RK, Goldstein JL (1995) cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem 270:1843–1849
Goddard I, Florin A, Mauduit C, Tabone E, Contard P, Bars R, Chuzel F, Benahmed M (2003) Alteration of lactate production and transport in the adult rat testis exposed in utero to flutamide. Mol Cell Endocrinol 206:137–146
Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343:281–299
Halestrap AP, Meredith D (2004) The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628
Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM (2005) Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology 64:992–1006
Hawkins RA, Mans AM, Davis DW (1986) Regional ketone body utilization by rat brain in starvation and diabetes. Am J Physiol Endocrinol Metab 250:E169–E178
Hugentobler SA, Humpherson PG, Leese HJ, Sreeman JM, Morris DG (2008) Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol Reprod Develop 75:496–503
Jones AR (1997) Metabolism of lactate by mature boar spermatozoa. Reprod Fertil Dev 9:227–232
Kim JH, Funahashi H, Niwa K, Okuda K (1993) Glucose equipment at different developmental stages of in vitro fertilized bovine embryos cultured in semi-defined medium. Theriogenology 39:875–886
Kito S, Ohta Y (2008) In vitro fertilization in inbred BALB/c mice II: effects of lactate, osmolarity and calcium on in vitro capacitation. Zygote 16:259–270
Kol S, Ben-Shlomo I, Ruutianinen K, Ando M, Davies-Hill TM, Rohan RM, Simpson IA, Adashi EY (1997) The midcycle increase in ovarian glucose uptake is associated with enhanced expression of glucose transporter 3. J Clin Invest 99:2274–2283
Leese HJ, Barton AM (1984) Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fert 72:9–13
Leese HJ, Barton AM (1985) Production of pyruvate by isolated mouse cumulus cells. J Exp Zool 234:231–236
Leese HJ, Tay JI, Reischl J, Downing SJ (2001) Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction 121:339–346
Martin KL, Leese HJ (1995) Role of glucose in mouse preimplantation embryo development. Mol Reprod Dev 40:436–443
Medrano A, Fernández-Novell JM, Ramió L, Alvarez J, Goldberg E, Montserrat Rivera M, Guinovart JJ, Rigau T, Rodríguez-Gil JE (2006) Utilization of citrate and lactate through a lactate dehydrogenase and ATP-regulated pathway in boar spermatozoa. Mol Reprod Dev 73:369–378
Meredith D, Christian HC (2008) The SLC16 monocarboxylate transporter family. Xenobiotica 38:1072–1106
Nagai A, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T (2010) Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta 31:126–133
Nandi S, Girish Kumar V, Manjunatha BM, Ramesh HS, Gupta PSP (2008) Follicular fluid concentrations of glucose, lactate and pyruvate in buffalo and sheep, and their effects on cultured oocytes, granulosa and cumulus cells. Theriogenology 69:186–196
Nishimoto H, Matsutani R, Yamamoto S, Takahashi T, Hayashi K-G, Miyamoto A, Hamano S, Tetsuka M (2006) Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicles and corpus luteum. J Endocrinol 188:111–119
Pellerin L, Pellegri G, Martin J-L, Magistretti PJ (1998) Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci USA 95:3990–3995
Roller RJ, Kinloch RA, Hiraoka BY, Li SS-L, Wassarman PM (1989) Gene expression during mammalian oogenesis and early embryogenesis: quantification of three messenger RNAs abundant in fully grown mouse oocytes. Development 106:251–261
Sugiura K, Pendola FL, Eppig JJ (2005) Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 279:20–30
Takahashi Y, First NL (1992) In vitro development of bovine one-cell embryos: influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 37:963–978
Thangaraju M, Carswell KN, Prasad PD, Ganapathy V (2009) Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition HDAC1/HDAC3. Biochem J 417:379–389
Vannuci SJ, Simpson IA (2003) Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab 285:E1127–E1134
Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP (1998) Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem 273:15920–15926
Yamanouchi H, Umezu T, Tomooka Y (2010) Reconstruction of oviduct and demonstration of the epithelial fate determination in mice. Biol Reprod 82:528–533
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven Press, New York, pp 189–317
Zhou J, Bievre M, Bondy CA (2000) Reduced GLUT1 expression in Igf1−/− null oocytes and follicles. Growth Horm IGF Res 10:111–117
Acknowledgments
This study is supported by grants from the Ministry of Education, Science, Sport, and Culture, Japan (No. 22590184 to T. I.) and The Uehara Memorial Foundation (to T. I.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2011_794_MOESM1_ESM.tif
Supplemental information-1. In situ hybridization analysis for mRNA expressions of GLUT1–GLUT5. Two sections (a, b) from the same mouse are hybridized for GLUT1 and GLUT3 (c and d are dark-field images of a and b). GLUT 1 and GLUT3 are expressed mainly in the proximal (ampulla, Amp) and distal (isthmus, Isth) regions, respectively. No significant signals are visible in the ovary. Bar 200 μm. Figures e–h show X-ray film images for GLUT2–GLUT5 mRNAs. The ovary, oviduct, and uterus are mounted on the glass slides together with tissues for positive control (K: kidney, L: liver, B: brain, M: muscle, I: intestine). Intense signals in the oviduct (indicated by arrows) are found only for GLUT3, with faint signals for GLUT4 in the oviduct. Each glass slide contains four sections serially cut from the female reproductive organ. (TIFF 1384 kb)
418_2011_794_MOESM2_ESM.tif
Supplemental information-2. Immunohistochemistry for GLUT1 in the ovary and oviduct. The GLUT1 immunoreactivity in the ovary is restricted to some vascular endothelia (arrows in a). In the oviduct (b), the GLUT1 immunoreactivity is found in the distal region of the ampulla (arrow) but not the proximal part of the ampulla (Amp), including the infundibulum (Inf). (TIFF 561 kb)
418_2011_794_MOESM3_ESM.tif
Supplemental information-3. In situ hybridization on a single section for LDH-A mRNA in the oviduct. Bright field (a) and dark field (b) images show an intense expression of LDH-A in the isthmus of oviduct and the luminal epithelium of uterus. Inf: infundibulum (TIFF 728 kb)
418_2011_794_MOESM4_ESM.tif
Supplemental information-4. Three serial paraffin sections from the vagina at different estrus stages are stained for periodic acid-Schiff (PAS) reaction (a–c), MCT1 (d–f), and GLUT1 (g–i). The immunoreactivity for MCT1 is more intense at met-estrus to di-estrus while the GLUT1 immunoreactivity increases in intensity at pro-estrus to estrus. (TIFF 1054 kb)
Rights and permissions
About this article
Cite this article
Kuchiiwa, T., Nio-Kobayashi, J., Takahashi-Iwanaga, H. et al. Cellular expression of monocarboxylate transporters in the female reproductive organ of mice: implications for the genital lactate shuttle. Histochem Cell Biol 135, 351–360 (2011). https://doi.org/10.1007/s00418-011-0794-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0794-2