Abstract
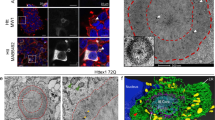
The stigmoid body (STB) is a neurocytoplasmic inclusion containing huntingtin-associated protein 1 (HAP1), an interactor of huntingtin, and its formation is induced by transfection of HAP1-cDNA into cultured cells. Although STB is believed to play a protective role in polyglutamine diseases, including Huntington’s disease and spinal and bulbar muscular atrophy, by sequestering the causative proteins, huntingtin and androgen receptor, respectively, its physiological function and formation remain poorly understood. Therefore, STB is occasionally confused with another cytoplasmic inclusion observed in polyglutamine diseases, the aggresome. Here we examined the subcellular dynamics of STB and compared it immunohistochemically and cytochemically with the aggresome in the rat brain and COS-7 or HeLa cells transfected with HAP1 and/or polyglutamine disease-associated genes. In time-lapse image analysis of HAP1-transfected cells, the HAP1-induced STB is formed from multiple fusions of small HAP1 inclusions characterized by vigorous cytoplasmic movement. In HAP1-transfected cells treated with a microtubule-depolymerizing drug, although the formation of small HAP1 inclusions was not affected, their fusion was critically inhibited. Immunohistochemistry and cytochemistry revealed the absence of association between STB and aggresomal markers, such as ubiquitin/proteasome, intermediate filaments, and the centrosome. Taken together, we concluded that STB is formed by a two-step process comprising microtubule-independent formation of small HAP1 inclusions and microtubule-dependent fusion of these inclusions, and that STB is distinct from pathological aggresomes.







Similar content being viewed by others
References
Amann-Zalcenstein D, Avidan N, Kanyas K, Ebstein RP, Kohn Y, Hamdan A, Ben-Asher E, Karni O, Mujaheed M, Segman RH, Maier W, Macciardi F, Beckmann JS, Lancet D, Lerer B (2006) AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet 14:1111–1119
Chan EY, Nasir J, Gutekunst CA, Coleman S, Maclean A, Maas A, Metzler M, Gertsenstein M, Ross CA, Nagy A, Hayden MR (2002) Targeted disruption of Huntingtin-associated protein-1 (Hap1) results in postnatal death due to depressed feeding behavior. Hum Mol Genet 11:945–959
Diamond MI, Robinson MR, Yamamoto KR (2000) Regulation of expanded polyglutamine protein aggregation and nuclear localization by the glucocorticoid receptor. Proc Natl Acad Sci USA 97:657–661
Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, Gleeson JG (2004) Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 75:979–987
Doering JE, Kane K, Hsiao YC, Yao C, Shi B, Slowik AD, Dhagat B, Scott DD, Ault JG, Page-McCaw PS, Ferland RJ (2008) Species differences in the expression of Ahi1, a protein implicated in the neurodevelopmental disorder Joubert syndrome, with preferential accumulation to stigmoid bodies. J Comp Neurol 511:238–256
Dragatsis I, Dietrich P, Zeitlin S (2000) Expression of the Huntingtin-associated protein 1 gene in the developing and adult mouse. Neurosci Lett 282:37–40
Dragatsis I, Zeitlin S, Dietrich P (2004) Huntingtin-associated protein 1 (Hap1) mutant mice bypassing the early postnatal lethality are neuroanatomically normal and fertile but display growth retardation. Hum Mol Genet 13:3115–3125
Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA (1997) Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet 6:2205–2212
Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, Shugart YY, Ruvolo M, Walsh CA (2004) Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36:1008–1013
Fujimuro M, Yokosawa H (2005) Production of antipolyubiquitin monoclonal antibodies and their use for characterization and isolation of polyubiquitinated proteins. Methods Enzymol 399:75–86
Fujinaga R, Kawano J, Matsuzaki Y, Kamei K, Yanai A, Sheng Z, Tanaka M, Nakahama K, Nagano M, Shinoda K (2004) Neuroanatomical distribution of Huntingtin-associated protein 1-mRNA in the male mouse brain. J Comp Neurol 478:88–109
Fujinaga R, Yanai A, Nakatsuka H, Yoshida K, Takeshita Y, Uozumi K, Zhao C, Hirata K, Kokubu K, Nagano M, Shinoda K (2007) Anti-human placental antigen complex X-P2 (hPAX-P2) anti-serum recognizes C-terminus of huntingtin-associated protein 1A common to 1B as a determinant marker for the stigmoid body. Histochem Cell Biol 128:335–348
Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES (1999) Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol 146:1239–1254
Gutekunst CA, Li SH, Yi H, Ferrante RJ, Li XJ, Hersch SM (1998) The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J Neurosci 18:7674–7686
Hirano K, Roth J, Zuber C, Ziak M (2002) Expression of a mutant ER-retained polytope membrane protein in cultured rat hepatocytes results in Mallory body formation. Histochem Cell Biol 117:41–53
Hirano K, Guhl B, Roth J, Ziak M (2009) A cell culture system for the induction of Mallory bodies: Mallory bodies and aggresomes represent different types of inclusion bodies. Histochem Cell Biol. doi: 10.1007/s00418-009-0598-9
Ingason A, Sigmundsson T, Steinberg S, Sigurdsson E, Haraldsson M, Magnusdottir BB, Frigge ML, Kong A, Gulcher J, Thorsteinsdottir U, Stefansson K, Petursson H, Stefansson H (2007) Support for involvement of the AHI1 locus in schizophrenia. Eur J Hum Genet 15:988–991
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898
Kamei K, Matsuzaki Y, Hanada K, Nagano M, Nakahama K, Shinoda K (2001) Distribution of HAP1 mRNA in the rat brain. Acta Anat Nippon 76(Suppl):A-91 (Abstract)
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10:524–530
Leask A, Obrietan K, Stearns T (1997) Synaptically coupled central nervous system neurons lack centrosomal gamma-tubulin. Neurosci Lett 229:17–20
Li XJ, Li SH (2005) HAP1 and intracellular trafficking. Trends Pharmacol Sci 26:1–3
Li XJ, Li SH, Sharp AH, Nucifora FC Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross CA (1995) A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378:398–402
Li SH, Gutekunst CA, Hersch SM, Li XJ (1998a) Association of HAP1 isoforms with a unique cytoplasmic structure. J Neurochem 71:2178–2185
Li SH, Gutekunst CA, Hersch SM, Li XJ (1998b) Interaction of huntingtin-associated protein with dynactin P150Glued. J Neurosci 18:1261–1269
Li Y, Chin LS, Levey AI, Li L (2002) Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking. J Biol Chem 277:28212–28221
Li SH, Yu ZX, Li CL, Nguyen HP, Zhou YX, Deng C, Li XJ (2003) Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington’s disease. J Neurosci 23:6956–6964
Liao M, Shen J, Zhang Y, Li SH, Li XJ, Li H (2005) Immunohistochemical localization of huntingtin-associated protein 1 in endocrine system of the rat. J Histochem Cytochem 53:1517–1524
Marcora E, Gowan K, Lee JE (2003) Stimulation of NeuroD activity by huntingtin and huntingtin-associated proteins HAP1 and MLK2. Proc Natl Acad Sci USA 100:9578–9583
McGuire JR, Rong J, Li SH, Li XJ (2006) Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem 281:3552–3559
Metzger S, Rong J, Nguyen HP, Cape A, Tomiuk J, Soehn AS, Propping P, Freudenberg-Hua Y, Freudenberg J, Tong L, Li SH, Li XJ, Riess O (2008) Huntingtin-associated protein-1 is a modifier of the age-at-onset of Huntington’s disease. Hum Mol Genet 17:1137–1146
Moulder KL, Onodera O, Burke JR, Strittmatter WJ, Johnson EM Jr (1999) Generation of neuronal intranuclear inclusions by polyglutamine-GFP: analysis of inclusion clearance and toxicity as a function of polyglutamine length. J Neurosci 19:705–715
Nagano M, Shinoda K (1994) Coexistence of the stigmoid body and estrogen receptor in some neuronal groups involved in rat reproductive functions. Brain Res 634:296–304
Nagaoka U, Kim K, Jana NR, Doi H, Maruyama M, Mitsui K, Oyama F, Nukina N (2004) Increased expression of p62 in expanded polyglutamine-expressing cells and its association with polyglutamine inclusions. J Neurochem 91:57–68
Nakamura K, Jeong SY, Uchihara T, Anno M, Nagashima K, Nagashima T, Ikeda S, Tsuji S, Kanazawa I (2001) SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet 10:1441–1448
Nozawa N, Yamauchi Y, Ohtsuka K, Kawaguchi Y, Nishiyama Y (2004) Formation of aggresome-like structures in herpes simplex virus type 2-infected cells and a potential role in virus assembly. Exp Cell Res 299:486–497
Onodera O, Burke JR, Miller SE, Hester S, Tsuji S, Roses AD, Strittmatter WJ (1997) Oligomerization of expanded-polyglutamine domain fluorescent fusion proteins in cultured mammalian cells. Biochem Biophys Res Commun 238:599–605
Page KJ, Potter L, Aronni S, Everitt BJ, Dunnett SB (1998) The expression of Huntingtin-associated protein (HAP1) mRNA in developing, adult and ageing rat CNS: implications for Huntington’s disease neuropathology. Eur J Neurosci 10:1835–1845
Prigge JR, Schmidt EE (2007) HAP1 can sequester a subset of TBP in cytoplasmic inclusions via specific interaction with the conserved TBP(CORE). BMC Mol Biol 8:76
Rong J, Li SH, Li XJ (2007) Regulation of intracellular HAP1 trafficking. J Neurosci Res 85:3025–3029
Roth J, Yam GH, Fan J, Hirano K, Gaplovska-Kysela K, Le Fourn V, Guhl B, Santimaria R, Torossi T, Ziak M, Zuber C (2008) Protein quality control: the who’s who, the where’s and therapeutic escapes. Histochem Cell Biol 129:163–177
Saliba RS, Munro PM, Luthert PJ, Cheetham ME (2002) The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci 115:2907–2918
Sheng Z, Yanai A, Fujinaga R, Kawano J, Tanaka M, Watanabe Y, Shinoda K (2003) Gonadal and adrenal effects on the glucocorticoid receptor in the rat hippocampus, with special reference to regulation by estrogen from an immunohistochemical view-point. Neurosci Res 46:205–218
Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K (2004) Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res 49:185–196
Sheng G, Chang GQ, Lin JY, Yu ZX, Fang ZH, Rong J, Lipton SA, Li SH, Tong G, Leibowitz SF, Li XJ (2006) Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med 12:526–533
Sheng G, Xu X, Lin YF, Wang CE, Rong J, Cheng D, Peng J, Jiang X, Li SH, Li XJ (2008) Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J Clin Invest 118:2785–2795
Shimohata T, Sato A, Burke JR, Strittmatter WJ, Tsuji S, Onodera O (2002) Expanded polyglutamine stretches form an ‘aggresome’. Neurosci Lett 323:215–218
Shimojo M (2008) Huntingtin regulates RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) nuclear trafficking indirectly through a complex with REST/NRSF-interacting LIM domain protein (RILP) and Dynactin p150Glued. J Biol Chem 283:34880–34886
Shinoda K, Mori S, Ohtsuki T, Osawa Y (1992) An aromatase-associated cytoplasmic inclusion, the “stigmoid body”, in the rat brain: I. Distribution in the forebrain. J Comp Neurol 322:360–376
Shinoda K, Nagano M, Osawa Y (1993) An aromatase-associated cytoplasmic inclusion, the “stigmoid body”, in the rat brain: II. Ultrastructure (with a review of its history and nomenclature). J Comp Neurol 329:1–19
Strnad P, Stumptner C, Zatloukal K, Denk H (2008) Intermediate filament cytoskeleton of the liver in health and disease. Histochem Cell Biol 129:735–749
Takeshita Y, Fujinaga R, Zhao C, Yanai A, Shinoda K (2006) Huntingtin-associated protein 1 (HAP1) interacts with androgen receptor (AR) and suppresses SBMA-mutant-AR-induced apoptosis. Hum Mol Genet 15:2298–2312
Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I (2003) Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1, 4, 5) triphosphate receptor type 1. Neuron 39:227–239
Tang TS, Tu H, Orban PC, Chan EY, Hayden MR, Bezprozvanny I (2004) HAP1 facilitates effects of mutant huntingtin on inositol 1, 4, 5-trisphosphate-induced Ca release in primary culture of striatal medium spiny neurons. Eur J Neurosci 20:1779–1787
Taylor JP, Tanaka F, Robitschek J, Sandoval CM, Taye A, Markovic-Plese S, Fischbeck KH (2003) Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet 12:749–757
Vadlamudi RK, Joung I, Strominger JL, Shin J (1996) p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem 271:20235–20237
Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE (2001) Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell 12:1393–1407
Webb JL, Ravikumar B, Rubinsztein DC (2004) Microtubule disruption inhibits autophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases. Int J Biochem Cell Biol 36:2541–2550
Zander C, Takahashi J, El Hachimi KH, Fujigasaki H, Albanese V, Lebre AS, Stevanin G, Duyckaerts C, Brice A (2001) Similarities between spinocerebellar ataxia type 7 (SCA7) cell models and human brain: proteins recruited in inclusions and activation of caspase-3. Hum Mol Genet 10:2569–2579
Zhao C, Fujinaga R, Tanaka M, Yanai A, Nakahama K, Shinoda K (2007) Region-specific expression and sex-steroidal regulation on aromatase and its mRNA in the male rat brain: immunohistochemical and in situ hybridization analyses. J Comp Neurol 500:557–573
Acknowledgments
We cordially thank Dr. Keith R. Yamamoto (School of Medicine, University of California, San Francisco, CA, USA) for providing HA-ARN127(Q65) plasmid, and Drs. Warren J. Strittmatter and James R. Burke (Duke University Medical Center, Durham, NC, USA) for the Q80-GFP plasmid. We are grateful to Dr. Makoto Inui (Yamaguchi University) for his advice on the preparation of stable transformant. We also thank Dr. Changjiu Zhao, Dr. Kumiko Yoshida, Mr. Chikahisa Matsuo, Mr. Jun Oba, and Mrs. Yumiko Matsuzaki for their technical assistance and administrative help. We would also like to acknowledge the DNA Core facility of the Center for Gene Research, Yamaguchi University, supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan and the technical expertise of the Institute for Biomedical Research and Education, Yamaguchi University Science Research Center. This study was supported by Grant-in-Aids for Young Scientists (B) (No. 17700333) and for Scientific Research on Priority Areas (No.19040020) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Grant-in-Aids for Scientific Research (C) (No.17500231) and for Young Scientists (Start-up) (No. 19800027) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujinaga, R., Takeshita, Y., Uozumi, K. et al. Microtubule-dependent formation of the stigmoid body as a cytoplasmic inclusion distinct from pathological aggresomes. Histochem Cell Biol 132, 305–318 (2009). https://doi.org/10.1007/s00418-009-0618-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0618-9