Abstract
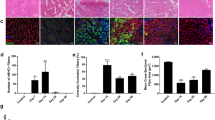
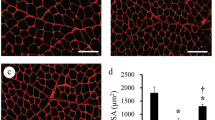
Tissue inflammation and multiple cellular responses in the compensatory enlarged plantaris (OP Plt) muscle induced by surgical ablation of synergistic muscles (soleus and gastrocnemius) were followed over 10 weeks after surgery. Contralateral surgery was performed in adult Wistar male rats. Cellular responses in muscle fibers, blood vessels and nerve fibers were analyzed by immunohistochemistry and electron microscopy. Severe muscle fiber damage and disappearance of capillaries associated with apparent tissue edema were observed in the peripheral portion of OP Plt muscles during the first week, whereas central portions were relatively preserved. Marked cell activation/proliferation was also mainly observed in peripheral portions. Similarly, activated myogenic cells were seen not only inside but also outside of muscle fibers. The former were likely satellite cells and the latter may be interstitial myogenic cells. One week after surgery, small muscle fibers, small arteries and capillaries and several branched-muscle fibers were evident in the periphery, thus indicating new muscle fiber and blood vessel formation. Proliferating cells were also detected in the nerve bundles in the Schwann cell position. These results indicate that the compensatory stimulated/enlarged muscle is a suitable model for analyzing multiple physiological cellular responses in muscle–nerve–blood vessel units under continuous stretch stimulation.







Similar content being viewed by others
References
Allbrook D (1981) Skeletal muscle regeneration. Muscle Nerve 4:234–245
Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, Freilinger M, Hoger H, Elbe-Burger A, Wachtler F (1999) Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 199:391–396
Bulfield G, Siller WG, Wight PA, Moore KJ (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81:1189–1192
Couteaux R, Mira JC, d’Albis A (1988) Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol Cell 62:171–182
Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J (2005) Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell 16:3323–3333
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9:255–267
Egginton S, Zhou AL, Brown MD, Hudlicka O (2001) Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res 49:634–646
Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528–1530
Grounds MD (1991) Towards understanding skeletal muscle regeneration. Pathol Res Pract 187:1–22
Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC (1999) Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401:390–394
Hansen-Smith F, Egginton S, Zhou AL, Hudlicka O (2001) Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. Microvasc Res 62:1–14
Hoffman EP, Morgan JE, Watkins SC, Partridge TA (1990) Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci 99:9–25
Hoshino S, Ohkoshi N, Ishii A, Shoji S (2002) The expression of alpha-dystrobrevin and dystrophin during skeletal muscle regeneration. J Muscle Res Cell Motil 23:131–138
Jackson KA, Mi T, Goodell MA (1999) Hematopoietic potential of stem cells isolated from murine skeletal muscle [see comments]. Proc Natl Acad Sci USA 96:14482–14486
Jankowski RJ, Deasy BM, Cao B, Gates C, Huard J (2002) The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci 115:4361–4374
LaBarge MA, Blau HM (2002) Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111:589–601
Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J (2000) Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol 150:1085–1100
Partridge T (2004) Reenthronement of the muscle satellite cell. Cell 119:447–448
Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J (2002) Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 157:851–864
Salah-Mohellibi N, Millet G, Andre-Schmutz I, Desforges B, Olaso R, Roblot N, Courageot S, Bensimon G, Cavazzana-Calvo M, Melki J (2006) Bone marrow transplantation attenuates the myopathic phenotype of a muscular mouse model of spinal muscular atrophy. Stem Cells 24:2723–2732
Schiaffino S, Bormioli SP, Aloisi M (1976) The fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Arch B Cell Pathol 21:113–118
Tajika Y, Sato M, Murakami T, Takata K, Yorifuji H (2007) VAMP2 is expressed in muscle satellite cells and up-regulated during muscle regeneration. Cell Tissue Res 328:573–581
Tamaki T, Akatsuka A (1994) Appearance of complex branched fibers following repetitive muscle trauma in normal rat skeletal muscle. Anat Rec 240:217–224
Tamaki T, Shiraishi T (1996) Characteristics of compensatory hypertrophied muscle in the rat: II. Comparison of histochemical and functional properties. Anat Rec 246:335–342
Tamaki T, Uchiyama S (1995) Absolute and relative growth of rat skeletal muscle. Physiol Behav 57:913–919
Tamaki T, Sekine T, Akatsuka A, Uchiyama S, Nakano S (1993) Three-dimensional cytoarchitecture of complex branched fibers in soleus muscle from mdx mutant mice. Anat Rec 237:338–344
Tamaki T, Akatsuka A, Tokunaga M, Uchiyama S, Shiraishi T (1996) Characteristics of compensatory hypertrophied muscle in the rat: I. Electron microscopic and immunohistochemical studies. Anat Rec 246:325–334
Tamaki T, Akatsuka A, Tokunaga M, Ishige K, Uchiyama S, Shiraishi T (1997) Morphological and biochemical evidence of muscle hyperplasia following weight-lifting exercise in rats. Am J Physiol 273:C246–C256
Tamaki T, Uchiyama S, Uchiyama Y, Akatsuka A, Roy RR, Edgerton VR (2001) Anabolic steroids increase exercise tolerance. Am J Physiol Endocrinol Metab 280:E973–E981
Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR (2002a) Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 157:571–577
Tamaki T, Akatsuka A, Yoshimura S, Roy RR, Edgerton VR (2002b) New fiber formation in the interstitial spaces of rat skeletal muscle during postnatal growth. J Histochem Cytochem 50:1097–1111
Tamaki T, Akatsuka A, Okada Y, Matsuzaki Y, Okano H, Kimura M (2003) Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp Cell Res 291:83–90
Tamaki T, Uchiyama Y, Okada Y, Ishikawa T, Sato M, Akatsuka A, Asahara T (2005) Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation 112:2857–2866
Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Wada M, Hoshi A, Ishikawa T, Akatsuka A (2007a) Clonal multipotency of skeletal muscle-derived stem cells between mesodermal and ectodermal lineage. Stem Cells 25:2283–2290
Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Wada M, Hoshi A, Akatsuka A (2007b) Synchronized reconstitution of muscle fibers, peripheral nerves and blood vessels by murine skeletal muscle-derived CD34(-)/45(-) cells. Histochem Cell Biol 128:349–360
Tamaki T, Uchiyama Y, Okada Y, Tono K, Nitta M, Hoshi A, Akatsuka A (2009) Anabolic-androgenic steroid does not enhance compensatory muscle hypertrophy but significantly diminish muscle damages in the rat surgical ablation model. Histochem Cell Biol. doi:10.1007/s00418-009-0584-2
Timson BF (1990) Evaluation of animal models for the study of exercise-induced muscle enlargement. J Appl Physiol 69:1935–1945
Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El Fahime M, D’Angelo MG, Caron NJ, Constantin G, Paulin D, Scarlato G, Bresolin N (2001) Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol 152:335–348
Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG (1999) Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg 65:22–26
Yamada S, Buffinger N, DiMario J, Strohman RC (1989) Fibroblast growth factor is stored in fiber extracellular matrix and plays a role in regulating muscle hypertrophy. Med Sci Sports Exerc 21:S173–S180
Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC Jr (2001) Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec 264:51–62
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research (B-12480012) from the Ministry of Education, Science and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2009_585_MOESM1_ESM.tif
Supplemental Figure 1. Basal lamina shape between large mature and small immature fibers presenting in Figure 5E. Individual basal lamina can be seen in both fibers (arrows). Satellite cells having small amounts of cytoplasm are also evident in large mature fibers (SC). Gaps in both fibers are also evident due to the periphery of unknown cells (asterisk). (TIFF 672 kb)
Rights and permissions
About this article
Cite this article
Tamaki, T., Uchiyama, Y., Okada, Y. et al. Multiple stimulations for muscle–nerve–blood vessel unit in compensatory hypertrophied skeletal muscle of rat surgical ablation model. Histochem Cell Biol 132, 59–70 (2009). https://doi.org/10.1007/s00418-009-0585-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0585-1