Abstract
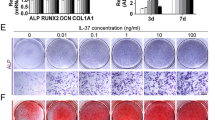
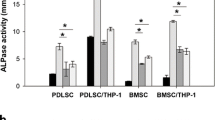
An intimate interplay exists between the bone and the immune system, which has been recently termed osteoimmunology. The activity of immune cells affects the intrinsic balance of bone mineralization and resorption carried out by the opposing actions of osteoblasts and osteoclasts. The aim of this study was to determine the possible interaction between inflammatory-induced conditions and matrix metalloproteinases-2,-9 (MMP-2,-9) synthesis and secretion by bone marrow-derived osteoprogenitor cells during advanced stages of osteogenesis. Rat bone marrow-derived mesenchymal stem cells (MSCs) were cultured in the presence of osteogenic supplements in order to direct the cells towards the osteogenic differentiation lineage. At the late stages of osteogenesis, assessed by histochemistry, immunohistochemistry and RT-PCR, cultures were exposed to pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) and interleukin-1alpha (IL-1α). Biochemical, histochemical and molecular biology techniques were used to discern the influence of pro-inflammatory cytokines on MMP-2,-9 synthesis and secretion. Results indicated that MMP-9 synthesis and secretion were significantly induced after exposure to the cytokines (TNF-α, IL-1α) treatment, while MMP-2 levels remained unchanged. These results indicate that in response to inflammatory processes, osteoblasts, in addition to osteoclasts, can also be involved and contribute to the process of active bone resorption by secretion and activation of MMPs.






Similar content being viewed by others
References
Aimes RT, Quigley JP (1995) Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270:5872–5876
Beck GR Jr. (2003) Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem 90:234–243
Bergmann U, Tuuttila A, Stetler-Stevenson WG, Tryggvason K (1995) Autolytic activation of recombinant human 72 kilodalton type IV collagenase. Biochemistry 34:2819–2825
Brown PD, Levy AT, Margulies IM, Liotta LA, Stetler-Stevenson WG (1990) Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res 50:6184–6191
Clowes JA, Riggs BL, Khosla S (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208:207–227
Everts V, Delaisse JM, Korper W, Beertsen W (1998) Cysteine proteinases and matrix metalloproteinases play distinct roles in the subosteoclastic resorption zone. J Bone Miner Res 13:1420–1430
Fujisaki K, Tanabe N, Suzuki N, Mitsui N, Oka H, Ito K, Maeno M (2006) The effect of IL-1alpha on the expression of matrix metalloproteinases, plasminogen activators, and their inhibitors in osteoblastic ROS 17/2.8 cells. Life Sci 78:1975–1982
Gepstein A, Shapiro S, Arbel G, Lahat N, Livne E (2002) Expression of matrix metalloproteinases in articular cartilage of temporomandibular and knee joints of mice during growth, maturation, and aging. Arthritis Rheum 46:3240–3250
Gepstein A, Arbel G, Blumenfeld I, Peled M, Livne E (2003) Association of metalloproteinases, tissue inhibitors of matrix metalloproteinases, and proteoglycans with development, aging, and osteoarthritis processes in mouse temporomandibular joint. Histochem Cell Biol 120:23–32
Ginaldi L, Di Benedetto MC, De Martinis M (2005) Osteoporosis, inflammation and ageing. Immun Ageing 2:14
Groft LL, Muzik H, Rewcastle NB, Johnston RN, Knauper V, Lafleur MA, Forsyth PA, Edwards DR (2001) Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer 85:55–63
Kahari VM, Saarialho-Kere U (1997) Matrix metalloproteinases in skin. Exp Dermatol 6:199–213
Kolkenbrock H, Orgel D, Hecker-Kia A, Zimmermann J, Ulbrich N (1995) Generation and activity of the ternary gelatinase B/TIMP-1/LMW-stromelysin-1 complex. Biol Chem Hoppe Seyler 376:495–500
Kolkenbrock H, Hecker-Kia A, Orgel D, Kinawi A, Ulbrich N (1996) Progelatinase B forms from human neutrophils. Complex formation of monomer/lipocalin with TIMP-1. Biol Chem 377:529–533
Lin SK, Kok SH, Kuo MY, Lee MS, Wang CC, Lan WH, Hsiao M, Goldring SR, Hong CY (2003) Nitric oxide promotes infectious bone resorption by enhancing cytokine-stimulated interstitial collagenase synthesis in osteoblasts. J Bone Miner Res 18:39–46
Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M (2008) Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40(1):46–62
Mizutani A, Sugiyama I, Kuno E, Matsunaga S, Tsukagoshi N (2001) Expression of matrix metalloproteinases during ascorbate-induced differentiation of osteoblastic MC3T3-E1 cells. J Bone Miner Res 16:2043–2049
Moll UM, Youngleib GL, Rosinski KB, Quigley JP (1990) Tumor promoter-stimulated Mr 92,000 gelatinase secreted by normal and malignant human cells: isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res 50:6162–6170
Morgan H, Hill PA (2005) Human breast cancer cell-mediated bone collagen degradation requires plasminogen activation and matrix metalloproteinase activity. Cancer Cell Int [electronic resource] 5:1
Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A (1994) Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci 732:31–41
Parikka V, Vaananen A, Risteli J, Salo T, Sorsa T, Vaananen HK, Lehenkari P (2005) Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biol 24(6):438–447
Patterson ML, Atkinson SJ, Knauper V, Murphy G (2001) Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett 503:158–162
Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21:1104–1117
Philip S, Bulbule A, Kundu GC (2004) Matrix metalloproteinase-2: mechanism and regulation of NF-kappaB-mediated activation and its role in cell motility and ECM-invasion. Glycoconj J 21:429–441
Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K (1994) High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol 124:1091–1102
Rifas L, Fausto A, Scott MJ, Avioli LV, Welgus HG (1994) Expression of metalloproteinases and tissue inhibitors of metalloproteinases in human osteoblast-like cells: differentiation is associated with repression of metalloproteinase biosynthesis. Endocrinology 134:213–221
Roodman GD (1999) Cell biology of the osteoclast. Exp Hematol 27:1229–1241
Saftig P, Hunziker E, Everts V, Jones S, Boyde A, Wehmeyer O, Suter A, von Figura K (2000) Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice. Adv Exp Med Biol 477:293–303
Sasaki T, Gohring W, Mann K, Maurer P, Hohenester E, Knauper V, Murphy G, Timpl R (1997) Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem 272:9237–9243
Srouji S, Maurice S, Livne E (2005) Microscopy analysis of bone marrow-derived osteoprogenitor cells cultured on hydrogel 3-D scaffold. Microsc Res Tech 66:132–138
Uchida M, Shima M, Shimoaka T, Fujieda A, Obara K, Suzuki H, Nagai Y, Ikeda T, Yamato H, Kawaguchi H (2000) Regulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) by bone resorptive factors in osteoblastic cells. J Cell Physiol 185:207–214
Wucherpfennig AL, Li YP, Stetler-Stevenson WG, Rosenberg AE, Stashenko P (1994) Expression of 92 kD type IV collagenase/gelatinase B in human osteoclasts. J Bone Miner Res 9:549–556
Ylisirnio S, Hoyhtya M, Makitaro R, Paaakko P, Risteli J, Kinnula VL, Turpeenniemi-Hujanen T, Jukkola A (2001) Elevated serum levels of type I collagen degradation marker ICTP and tissue inhibitor of metalloproteinase (TIMP) 1 are associated with poor prognosis in lung cancer. Clin Cancer Res 7:1633–1637
Acknowledgments
This work has been supported by the Israel Ministry of Commerce, Magnet Grant No. 2007992 and by Joint-Brookdale Gerontology Institute, and a grant from the Krol Founadtion of Barnegat NJ. USA to AZR. The excellent technical work of Mrs Pessia Schenzer and Mrs Janette Zavin is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben David, D., Reznick, A.Z., Srouji, S. et al. Exposure to pro-inflammatory cytokines upregulates MMP-9 synthesis by mesenchymal stem cells-derived osteoprogenitors. Histochem Cell Biol 129, 589–597 (2008). https://doi.org/10.1007/s00418-008-0391-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-008-0391-1