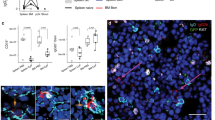
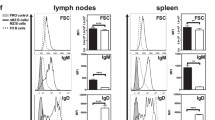
Abstract
The role of the spleen in B memory cell development and maintenance is attracting increased attention. Studies in mice and rats have indicated that memory functions are associated with large B cells residing in the marginal zone (MZ) of the spleen. Although the cellular composition of the MZ is relatively well known in these species, controversies exist about the function of MZ B cells, their dependence on the presence of the spleen and the stage at which their development branches from that of recirculating follicular B cells. Additional confusion has arisen with respect to MZ B cells in humans, because the microscopic anatomy of the human splenic MZ differs decisively from that of rodents. Several recent publications indicate that the functional and migratory properties of human MZ B cells may be species-specific. The hypothesis derived from these publications and from our immunohistological observations implies that at least a major number of human splenic CD27+ MZ B cells are migratory. Phenotypic data suggest a recirculation pathway between the spleen and mucosal tissues in humans.






Similar content being viewed by others
References
Amlot PL, Hayes AE (1985) Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Lancet I:1008–1011
Balazs M, Martin F, Zhou T, Kearney JF (2002) Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17:341–352
Brelinska R, Pilgrim C (1983) Macrophages and interdigitating cells: their relationship to migrating lymphocytes in the white pulp of rat spleen. Cell Tissue Res 233:671–688
Breukels MA, Zandvoort A, Rijkers GT, Lodewijk ME, Klok PA, Harms G, Timens W (2005) Complement dependency of splenic localization of pneumococcal polysaccharide and conjugate vaccines. Scand J Immunol 61:322–328
Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ (1997) Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 151:97–110
Buffet PA, Milon G, Brousse V, Correas JM, Dousset B, Couvelard A, Kianmanesh R, Farges O, Sauvanet A, Paye F, Ungeheuer MN, Ottone C, Khun H, Fiette L, Guigon G, Huerre M, Mercereau-Puijalon O, David PH (2006) Ex-vivo perfusion of human spleens maintains clearing and processing functions. Blood 107:3745–3752
Castagnola E, Fioredda F (2003) Prevention of life-threatening infections due to encapsulated bacteria in children with hyposplenia or asplenia: a brief review of current recommendations for practical purposes. Eur J Haematol 71:319–326
Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG (2004) Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol 5:713–720
Claassen E, Kors N, Dijkstra CD, van Rooijen N (1986) Marginal zone of the spleen and the development of specific antibody-forming cells against thymus-dependent and thymus-independent type-2 antigens. Immunology 57:399–403
Coil JA, Dickerman JD, Boulton E (1978) Increased susceptibility of splenectomized mice to infection after exposure to an aerosolized suspension of type III Streptococcus pneumoniae. Infect Immun 21:412–416
Dammers PM, Kroese FG (2005) Recruitment and selection of marginal zone B cells is independent of exogenous antigens. Eur J Immunol 35:2089–2099
Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FGM (2000) Most marginal zone B cells in rat express germline encoded IgVH genes and are ligand selected. J Immunol 165:6156–6169
De Vos AF, van Riel DA, van Meurs M, Brok HP, Boon L, Hintzen RQ, Claassen E, t’ Hart BA, Laman JD (2005) Severe T-cell depletion from the PALS leads to altered spleen composition in common marmosets with experimental autoimmune encephalomyelitis (EAE). J Neuroimmunol 161:29–39
Dijkstra CD, Döpp EA, Joling P, Kraal G (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies. Immunology 54:589–599
Di Sabatino A, Rosado MM, Ciccocioppo R, Cazzola P, Morera R, Corazza GR, Carsetti R (2005) Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 100:1788–1795
Dunn-Walters DK, Isaacson PG, Spencer J (1995) Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (mgz) B cells suggests that the mgz of human spleen is a reservoir of memory B cells. J Exp Med 182:559–566
Dunn-Walters DK, Isaacson PG, Spencer J (1996) Sequence analysis of rearranged IgVH genes from microdissected human Peyer’s patch marginal zone B cells. Immunology 88:618–624
Girkontaite I, Sakk V, Wagner M, Borggrefe T, Tedford K, Chun J, Fischer KD (2004) The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in marginal sinus organization. J Exp Med 200:1491–1501
Gray D, MacLennan ICM, Bazin H, Khan M (1982) Migrant μ+δ+and static μ+δ- B lymphocyte subsets. Eur J Immunol 12:564–569
Iizuka T, Tanaka T, Suematsu M, Miura S, Watanabe T, Koike R, Ishimura Y, Ishii H, Miyasaka N, Miyasaka M (2000) Stage-specific expression of mucosal addressin cell adhesion molecule-1 during embryogenesis in rats. J Immunol 164:2463–2471
Kanayama N, Cascalho M, Ohmori H (2005) Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone. J Immunol 174:1438–1445
Klein U, Rajewsky K, Küppers R (1998) Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 188:1679–1689
Koppel EA, Wieland CW, van den Berg VC, Litjens M, Florquin S, van Kooyk Y, van der Poll T, Geijtenbeek TB (2005) Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur J Immunol 35:2962–2969
Kraal G (1992) Cells in the marginal zone of the spleen. Int Rev Cytol 132:31–74
Kraal G, Janse A (1986) Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology 58:665–669
Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC (1995) Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol 147:763–771
Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R (2003) Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med 197:939–945
Kumararatne DS, MacLennan ICM (1981) Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur J Immunol 11:865–869
Kumararatne DS, Bazin H, MacLennan ICM (1981) Marginal zones: the major B cell compartment of rat spleens. Eur J Immunol 11:858–864
Liu Y-J, Oldfield S, MacLennan ICM (1988) Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur J Immunol 18:355–362
Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM (1991) Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol 21:2951–2962
Llende M, Santiago-Delpin EA, Lavergne J (1986) Immunobiological consequences of splenectomy: a review. J Surg Res 40:85–94
Lopes-Carvalho T, Kearney JF (2004) Development and selection of marginal zone B cells. Immunol Rev 197:192–205
Lu TT, Cyster JG (2002) Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297:409–412
MacLennan ICM, Liu Y-J (1991) Marginal zone B cells respond both to polysaccharide antigens and protein antigens. Res Immunol 142:346–351
MacLennan ICM, Gulbranson-Judge A, Toellner K-M, Casamayor-Palleja M, Chan E, Sze DM-Y, Luther SA, Acha Orbea H (1997) The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev 156:53–66
Martin F, Kearney JF (2002) Marginal zone B cells. Nat Rev Immunol 2:323–335
Mebius RE, Kraal G (2005) Structure and function of the spleen. Nat Rev Immunol 5:606–616
Mond JJ, Lees A, Snapper CM (1995) T cell-independent antigens type 2. Annu Rev Immunol 13:655–692
Mukhopadhyay S, Chen Y, Sankala M, Peiser L, Pikkarainen T, Kraal G, Tryggvason K, Gordon S (2006) MARCO, an innate activation marker of macrophages, is a class A scavenger receptor for Neisseria meningitidis. Eur J Immunol 36:940–949
Nieuwenhuis P, Ford WL (1976) Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell Immunol 23:254–267
Pabst O, Förster R, Lipp M, Engel H, Arnold HH (2000) NKX2.3 is required for MAdCAM-1 expression and homing of lymphocytes in spleen and mucosa-associated lymphoid tissue. EMBO J 19:2015–2023
Pillai S, Cariappa A, Moran ST (2005) Marginal zone B cells. Annu Rev Immunol 23:161–196
Spencer J, Finn T, Pulford KAF, Mason DY, Isaacson PG (1985) The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol 62:607–612
Steiniger B, Barth P (2000) Microanatomy and function of the spleen. Adv Anat Embryol 151:1–100
Steiniger B, Barth P, Herbst B, Hartnell A, Crocker PR (1997) The species-specific structure of microanatomical compartments in the human spleen: Strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology 92:307–316
Steiniger B, Hellinger A, Barth P (2001) The perifollicular and marginal zones of the human splenic white pulp: do fibroblasts guide lymphocyte immigration? Am J Pathol 159:501–512
Steiniger B, Rüttinger L, Barth PJ (2003) The three-dimensional structure of human splenic white pulp compartments. J Histochem Cytochem 51:655–663
Steiniger B, Timphus E-M, Jacob R, Barth PJ (2005) CD27+ B cells in human lymphatic organs: re-evaluating the splenic marginal zone. Immunology 116:429–442
Suarez F, Lortholary O, Hermine O, Lecuit M (2006) Infection-associated lymphomas derived from marginal-zone B-cells: a model of antigen-driven lymphoproliferations. Blood 107:3034–3044
Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE (1998) Identification of functional human splenic memory cells by expression of CD148 and CD27. J Exp Med 188:1691–1703
Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C (1999) Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood 93:226–234
Timens W, Poppema S (1985) Lymphocyte compartments in human spleen. An immunohistologic study in normal spleens and noninvolved spleens in Hodgkin’s disease. Am J Pathol 120:443–454
Van den Oord JJ, de Wolf-Peeters C, Desmet VJ (1986) The marginal zone in the human reactive lymph node. Am J Clin Pathol 86:475–479
Van Krieken JHJM, te Velde J (1986) Immunohistology of the human spleen: an inventory of the localization of lymphocyte subpopulations. Histopathology 10:285–294
Van Krieken JHJM, te Velde J (1988) Normal histology of the human spleen. Am J Surg Pathol 12:777–785
Van Krieken JHJM, von Schilling C, Kluin PM, Lennert K (1989) Splenic marginal zone lymphocytes and related cells in the lymph node: a morphologic and immunohistochemical study. Hum Pathol 20:320–325
Vugmeyster Y, Seshasayee D, Chang W, Storn A, Howell K, Sa S, Nelson T, Martin F, Grewal I, Gilkerson E, Wu B, Thompson J, Ehrenfels BN, Ren S, Song A, Gelzleichter TR, Danilenko DM (2006) A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys. Am J Pathol 168:476–489
Weller S, Braun MC, Tan B, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova J-L, Reynaud C-A, Weill J-C (2004) Blood IgM “memory” B cells are circulating splenic marginal zone B cells in humans. Blood 104:3647–3654
Weller S, Reynaud CA, Weill JC (2005) Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol 26:85–89
Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K (2005) Evidence of marginal-zone B cell-positive selection in spleen. Immunity 23:297–308
Wong WY, Powars DR, Chan L, Hiti A, Johnson C, Overturf G (1992) Polysaccharide encapsulated bacterial infection in sickle cell anemia: a thirty year epidemiologic experience. Am J Hematol 39:176–182
Acknowledgments
We thank Michael Bette for arranging the figures for print. This work was supported by grant Ste 360/10-1 of the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steiniger, B., Timphus, E.M. & Barth, P.J. The splenic marginal zone in humans and rodents: an enigmatic compartment and its inhabitants. Histochem Cell Biol 126, 641–648 (2006). https://doi.org/10.1007/s00418-006-0210-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-006-0210-5