Abstract
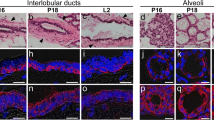
We examined the expression and immunolocalization of water-channel aquaporins in the mammary gland by reverse transcriptase polymerase chain reaction (RT-PCR), immunoblotting, and immunohistochemistry. RT-PCR and immunoblotting revealed the expression of aquaporin-1 (AQP1) and AQP3 in the lactating rat mammary gland. AQP3 was detected in the alveolar epithelium and duct system whereas AQP1 was found in the capillaries and venules. AQP3 was present in the basolateral membrane of secretory epithelial cells and intralobular and interlobular duct epithelial cells. The main duct near the orifice in the nipple, which is comprised of a stratified epithelium, bore AQP3 in its basal and intermediate layers. AQP1 was located in both the apical and basolateral membranes of capillary and venule endothelia. AQP3 was not detected in virgin females. AQP3 was found in some differentiating mammary epithelial cells in the pregnant rat. AQP1 was present in capillaries and venules in the differentiating mammary gland of the pregnant rat and in the mammary fat pad of virgin females. We found a similar distribution of AQP1 and AQP3 in the mouse. AQP1 and AQP3 seem to play roles in the synthesis and/or secretion of milk.








Similar content being viewed by others
References
Agre P, Sasaki S, Chrispeels MJ (1993) Aquaporins: a family of water channel proteins. Am J Physiol Renal Physiol 265:F461
Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S (2002) Aquaporin water channels—from atomic structure to clinical medicine. J Physiol (Lond) 542:3–16
Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S (1993) Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361:549–552
Garton GA (1963) The composition and biosynthesis of milk lipids. J Lipid Res 4:237–254
Ishibashi K, Sasaki S, Fushimi K, Yamamoto T, Kuwahara M, Marumo F (1997) Immunolocalization and effect of dehydration on AQP3, a basolateral water channel of kidney collecting ducts. Am J Physiol Renal Physiol 272:235–241
Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, Nishida M, Nishizawa H, Matsuda M, Takahashi M, Hotta K, Nakamura T, Yamashita S, Tochino Y, Matsuzawa Y (2000) Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 275:20896–20902
Loo DDF, Zeuthen T, Chandy G, Wright EM (1996) Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA 93:13367–13370
Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS (2000) Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci USA 97:4386–4391
Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K (1999a) Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res 295:513–521
Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K (1999b) Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J Histochem Cytochem 47:1275–1286
Matsuzaki T, Tajika Y, Suzuki T, Aoki T, Hagiwara H, Takata K (2003) Immunolocalization of water channel, aquaporin-5 (AQP5) in the rat digestive system. Arch Histol Cytol 66:307–315
Nejsum LN, Kwon T-H, Jensen UB, Fumagalli O, Frøkiær J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S (2002) Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci USA 99:511–516
Nielsen S, Smith BL, Christensen EI, Agre P (1993) Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci USA 90:7275–7279
Nielsen S, Frøkiær J, Marples D, Kwon T-H, Agre P, Knepper MA (2002) Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82:205–244
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus Oocytes expressing red cell CHIP28 protein. Science 256:385–387
Sabolić I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D (1992) Localization of the CHIP28 water channel in rat kidney. Am J Physiol Cell Physiol 263:1225–1233
Shennan DB, Peaker M (2000) Transport of milk constituents by the mammary gland. Physiol Rev 80:925–951
Shin BC, Suzuki T, Tanaka S, Kuraoka A, Shibata Y, Takata K (1996) Connexin 43 and the glucose transporter, GLUT1, in the ciliary body of the rat. Histochem Cell Biol 106:209–214
Smith BL, Agre P (1991) Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem 266:6407–6415
Song Y, Sonawane N, Verkman AS (2002) Localization of aquaporin-5 in sweat glands and functional analysis using knockout mice. J Physiol 541:561–568
Tajika Y, Matsuzaki T, Suzuki T, Aoki T, Hagiwara H, Tanaka S, Kominami E, Takata K (2002) Immunohistochemical characterization of the intracellular pool of water channel aquaporin-2 in the rat kidney. Anat Sci Int 77:189–195
Takata K, Fujikura K, Suzuki M, Suzuki T, Hirano H (1997) GLUT1 glucose transporter in the lactating mammary gland in the rat. Acta Histochem Cytochem 30:623–628
Takata K, Matsuzaki T, Tajika Y (2004) Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 39:1–83
Zhang R, Skach W, Hasegawa H, Van Hoek AN, Verkman AS (1993) Cloning, functional analysis and cell localization of a kidney proximal tubule water transporter homologous to CHIP28. J Cell Biol 120:359–369
Acknowledgements
We thank Y. Takahashi-Tajika and M. Kusama for assistance. This work was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Science and Technology of Japan, and by a scientific grant from the Kazato Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuzaki, T., Machida, N., Tajika, Y. et al. Expression and immunolocalization of water-channel aquaporins in the rat and mouse mammary gland. Histochem Cell Biol 123, 501–512 (2005). https://doi.org/10.1007/s00418-005-0753-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0753-x