Abstract
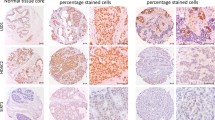
Histone H10 is a linker histone subvariant present in tissues of low proliferation rate. It is supposed to participate in the expression and maintenance of the terminal differentiation phenotype. The aim of this work was to study histone H10 distribution in human breast carcinoma and its relationship with the processes of proliferation and differentiation. Most of the cells in carcinomas of moderate and high level of differentiation expressed histone H10 including cells invading connective and adipose tissues. In low differentiated tumours, the number of H10 expressing cells was considerably lower. Staining of myoepithelial cells, when seen, and of stromal fibroblasts was variable. The metastatic malignant cells in the lymph nodes also accumulated H10 but lymphocytes were always negative. All immunopositive malignant cells exhibited signs of polymorphism. Double H10/Ki-67 staining showed that the growth fraction in more differentiated tumours belonged to the H10-positive cells, while in poorly differentiated carcinomas it also included a cell subpopulation not expressing H10. If expressed, p27Kip1 was always found in H10-positive cells. These findings are inconsistent with the widespread view that histone H10 is expressed only in terminally differentiated cells. Rather, they suggest that the protein is expressed in cells in a prolonged intermitotic period irrespective of their level of differentiation. Double H10/Ki-67 immunostaining could be a useful tool in studying the growth fraction in tumours.



Similar content being viewed by others
References
Balhorn R, Chalkley R, Granner D (1972) Lysine-rich histone phosphorylation. A positive correlation with cell replication. Biochemistry 11:1094–1098
Banchev T, Srebreva L, Zlatanova J, Tsanev R (1988) Immunofluorescent localization of histone H10 in the nuclei of proliferating and differentiating Friend cells. Exp Cell Res 177:1–8
Barbareschi M (1999) p27 expression, a cyclin dependent kinase inhibitor in breast carcinoma. Adv Clin Path 3:119–127
Barnes A, Pinder SE, Bell JA, Paish EC, Wencyk PM, Robertson JFR, Elston CW, Ellis IO (2003) Expression of p27kip1 in breast cancer and its prognostic significance. J Pathol 201:451–459
Benjamin WB (1971) Selective in vitro methylation of rat chromatin associated histone after partial hepatectomy. Nature New Biol 234:18–20
Boix J, Ruiz-Carrillo (1992) Increased histone H1° expression in differentiating mouse erythroleukemia cells is related to decreased cell proliferation. Exp Cell Res 201:531–534
Cariou S, Catzavelos C, Slingerland JM (1998) Prognostic implications of expression of the cell cycle inhibitor protein p27Kip1. Breast Cancer Res Treat 52:29–41
D’Incalci M, Allavena P, Wu R, Bonner W (1986) H1 variant synthesis in proliferating and quiescent human cells. Eur J Biochem 154:273–279
Doenecke D, Alonso A (1996) Organization and expression of the developmentally regulated H10 histone gene in vertebrates. Int J Dev Biol 40:395–401
Eisen H, Gjerset R, Hasthorpe S (1981) Distribution and regulation of histone H10 in rodents. In: Lloyd, Rees D (eds) Cellular controls in differentiation. Academic press, New York, 215–230
Elston CW, Ellis IO (1998) Assessment of histological grade. In: Elston CW, Ellis IO (eds) The breast, vol 13. Churchill Livingstone, Edinburgh, pp 356–384
Fedoseeva G, Srebreva L, Zlatanova J, Tsanev R (1983) Dynamics of H10 content in rat liver after partial hepatectomy. Int J Biochem 15:1489–1491
Gabrielli F, Aden D, Carrel S, von Bahr C, Rane A, Angeletti C, Hancock R (1985) Histone complements of human tissues, carcinomas, and carcinoma-derived cell lines. Mol Cell Biochem 65:57–66
García-Segura LM, Luquin S, Martinez P, Casas MT, Suau P (1993) Differential expression and gonadal hormone regulation of histone H10 in the developing and adult rat brain. Dev Brain Res 73:63–70
Garrard WT, Bonner J (1974) Changes in chromatin proteins during liver regeneration. J Biol Chem 249:5570–5579
Gjerset R, Gorka C, Hasthorpe S, Lawrence J, Eisen H (1982) Developmental and hormonal regulation of protein H1 degrees in rodents. Proc Natl Acad Sci USA 79:2333–2337
Gorka C, Lawrence J, Khochbin S (1995) Variation of H10 content throughout the cell cycle in regenerating rat liver. Exp Cell Res 217:528–533
Johns EW (1964) Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J 92:55–59
Keppel F, Allet B, Eisen H (1977) Appearance of a chromatin protein during the erythroid differentiation of Friend virus-transformed cells. Proc Natl Acad Sci USA 74:653–656
Khochbin S, Chabanas A, Albert P, Lawrence J (1989) Flow cytofluorimetric determination of protein distribution throughout the cell cycle. Cytometry 10:484–489
Koyama M, Kurotaki H, Yagihashi N, Aizawa S, Sugai M, Kamata Y, Oyama T, Yagihashi S (1997) Immunohistochemical assessment of proliferative activity in mammary adenomyoepithelioma. Histopathology 31:134–139
Lafarga M, García-Segura LM, Rodriguez JR, Suau P (1995) Expression of histone H10 in transcriptionally activated supraoptic neurons. Mol Brain Res 29:317–324
LaRue H, Bissonnette E, Belanger L (1983) Histone H10 expression during developmental growth of rat liver. Can J Biochem Cell Biol 6:1197–1200
Lea MA (1983) Nuclear proteins of tumours. Int J Biochem 15:767–770
Lea MA (1987) Relationship of H10 histone to differentiation and cancer. Cancer Biochem Biophys 9:199–209
Lea MA, Youngworth LA, Morris HP (1974) Acid soluble nuclear proteins of rat liver: differential absorbance of bound dyes and changes in neoplasia. Biochem Biophys Res Commun 58:862–867
Lennox R (1986) Murine erythroblasts do not contain histone H10. Dev Biol 118:319–323
Lennox R, Cohen L (1983) The histone H1 complements of dividing and nondividing cells of the mouse. J Biol Chem 258:262–268
Lindner H, Wurm M, Dirschlmayer A, Sarg B, Helliger W (1993) Application of high-performance capillary electrophoresis to the analysis of H1 histone. Electrophoresis 14:480–485
Lindner H, Helliger W, Sarg B, Meraner C (1995) Effect of buffer composition on the migration order and separation of histone H1 subtypes. Electrophoresis 16:604–610
Lindner H, Sarg B, Helliger W (2003) Capillary electrophoresis analysis of histones, histone variants, and their post-translationally modified forms: a review. J Capillary Electrophor 8:59–67
Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW (1999) p27Kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154:313–323
Mannironi C, Rossi V, Biondi A, Ubezio P, Masera G, Barbui T, D’Incalci M (1987) Histone H10 is synthesized by human lymphocytic leukemia cells but not by normal lymphocytes. Blood 70:1203–1207
Maraldi N, Cocco L, Papa S, Capitani S, Mazzotti G, Manzoli F (1978) Presence of H10 histone in human CLL lymphocytes. IRCS Med Sci 6:78–81
Marion C, Roche J, Roux B, Gorka C (1985) Differences in the condensation of chromatin by individual subfractions of histone H1: implications for the role of H10 in the structural organization of chromatin. Biochemistry 24:6328–6335
Marks D, Kanefsky T, Keller B, Marks A (1975) The presence of histone H10 in human tissues. Cancer Res 35:886–889
Marsh WH, Fitzgerald PJ (1973) Pancreas acinar cell regeneration. XIII. Histone synthesis and modification. Fed Proc 32:2119–2125
Medvedev ZA, Medvedeva MN (1980a) A group of H1 histone satellite acid-soluble non-histone chromatin proteins. FEBS Lett 112:35–38
Medvedev ZA, Medvedeva MN (1980b) High H10/H1 histone ratio in spontaneous hepatomas in aging mice. IRCS Med Sci 8:431–435
Musgrove EA, Davison EA, Ormandy CJ (2004) Role of the CDK inhibitor p27 (Kip1) in mammary development and carcinogenesis: insight from knockout mice. J Mammary Glad Biol Neoplasia 9:55–65
Osborne H, Chabanas A (1984) Kinetics of histone H10 accumulation and commitment to differentiation in murine erythroleukemia cells. Exp Cell Res 152:449–458
Panyim S, Chalkley R (1969a) A new histone found only in mammalian tissues with little cell division. Biochem Biophys Res Commun 37:1042–1049
Panyim S, Chalkley R (1969b) High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys 130:337–346
Rønnov-Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76:69–125
Roche J, Gorka C, Goeltz P, Lawrence J (1985) Association of histone H10 with a gene repressed during liver development. Nature 314:197–198
Said JW, Shintaku IP, Pinkus GS (1988) Immunohistochemical staining for terminal deoxynucleotidyl transferase (TDT). An enhanced method in routinely processed formalin-fixed tissue sections. Am J Clin Pathol 89:649–652
Sarg B, Helliger W, Hoertnagl B, Puschendorf B, Lindner H (1999) The N-terminally acetylated form of mammalian histone H10, but not that of avian histone H5, increases with age. Arch Biochem Biophys 372:333–339
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322
Smith B, Harris M, Sigournay C, Mayes E, Bustin M (1984) A survey of H10 - and H5-like protein structure and distribution in higher and lower eukaryotes. Eur J Biochem 138:309–317
Tsanev R, Hadjiolov D (1978) Chromosomal proteins in hepatocarcinogenesis. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol 91:237–247
Valiron O, Gorka C (1997) Histone H10 expression is restricted to progenitor cells during human hematopoiesis. Eur J Cell Biol 72:39–45
van Helden PD (1985) Histone H10: a maintainer of the differentiated cell state? Int J Biochem 17:381–385
van Holde KE (1988) Chromatin. Springer, Berlin Heidelberg New York
van der Loss CM, Becker AE, van der Oord JJ (1993) Practical suggestions for successful immunoenzyme double staining experiments. Histochem J 25:1–13
van der Loss CM (1999) Immunoenzyme multiple staining methods. Springer, BIOS Scientific Publisher LTD, Oxford
Varricchio F (1979) H1 histones of various human organs and tumours. Exp Mol Pathol 31:361–367
Varricchio F, Mabogunje O, Kim D, Fortner JG, Fitzgerald PJ (1977) Pancreas acinar cell regeneration and histone (H1 and H10) modifications after partial pancreatectomy or after a protein-free ethionine regimen. Cancer Res 37:3964–3969
Zlatanova J, Doenecke D (1994) Histone H10: a major player in cell differentiation? FASEB J 8:1260–1268
Acknowledgements
The skilful technical assistance of Mrs. Milena Petkova is gratefully acknowledged. This study was supported by grants from the Swedish Research Council (grant no. 349-2001-6688) and Bulgarian National Science Fund (grant no. K-906/1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00418-005-0084-y
Rights and permissions
About this article
Cite this article
Kostova, N.N., Srebreva, L.N., Milev, A.D. et al. Immunohistochemical demonstration of histone H10 in human breast carcinoma. Histochem Cell Biol 124, 435–443 (2005). https://doi.org/10.1007/s00418-005-0052-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0052-6