Abstract
Purposes
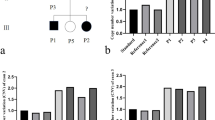
North Carolina macular dystrophy (NCMD) is a rare autosomal dominant inherited disorder characterized by macular impairment with a variety of phenotypic manifestations. The aims of this study were to assess the clinical features of a Chinese family with NCMD and to identify the underlying genetic cause of the disease.
Methods
Three patients from a Chinese family were included in this study. Detailed ophthalmological examinations were performed, including best corrected visual acuity (BCVA), slit lamp, dilated indirect ophthalmoscopy, fundus photography, optical coherence tomography (OCT), fundus autofluorescence, full-field electroretinography (ERG), and electrooculography (EOG). Genomic DNA was extracted from peripheral blood samples. Whole-genome sequencing and long-read genome sequencing were applied to detect the pathogenic variants. Sanger sequencing was performed to confirm the breakpoints.
Results
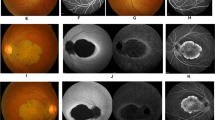
All three patients had macular involvement ranging from patchy yellowish-white lesions to big-area thinning, which are typical for NCMD. The BCVA ranged from 20/50 to 20/20. OCT revealed varying degrees of macular structure disorganization. The ERG responses were normal, and the Arden ration of the EOG was reduced. A novel 134.6 kb (g.99932464-100067110dup) tandem duplication on chromosome 6 (NC_000006.11) encompassing the entire CCNC and PRDM13 genes and a DNase 1 hypersensitivity site in the MCDR1 locus was identified.
Conclusion
A novel large tandem duplication in MCDR1 locus was confirmed in a Chinese family with NCMD with a variety of macular phenotypes.





Similar content being viewed by others
References
Lefler WH, Wadsworth JA, Sidbury JB Jr (1971) Hereditary macular degeneration and amino-aciduria. Am J Ophthalmol 71:224–230
Small KW (1989) North Carolina macular dystrophy, revisited. Ophthalmology 96:1747–1754. https://doi.org/10.1016/s0161-6420(89)32655-8
Kiernan DF, Shah RJ, Hariprasad SM, Grassi MA, Small KW, Kiernan JP, Mieler WF (2011) Thirty-Year follow-up of an African American family with macular dystrophy of the retina, locus 1 (North Carolina macular dystrophy). Ophthalmology 118:1435–1443. https://doi.org/10.1016/j.ophtha.2010.10.041
Reichel MB, Kelsell RE, Fan J, Gregory CY, Evans K, Moore AT, Hunt DM, Fitzke FW, Bird AC (1998) Phenotype of a British North Carolina macular dystrophy family linked to chromosome 6q. Br J Ophthalmol 82:1162–1168. https://doi.org/10.1136/bjo.82.10.1162
Small KW, Weber JL, Roses A, Lennon F, Vance JM, Pericak-Vance MA (1992) North Carolina macular dystrophy is assigned to chromosome 6. Genomics 13:681–685. https://doi.org/10.1016/0888-7543(92)90141-e
Small KW, Puech B, Mullen L, Yelchits S (1997) North Carolina macular dystrophy phenotype in France maps to the MCDR1 locus. Mol Vis 3:1
Michaelides M, Johnson S, Tekriwal AK, Holder GE, Bellmann C, Kinning E, Woodruff G, Trembath RC, Hunt DM, Moore AT (2003) An early-onset autosomal dominant macular dystrophy (MCDR3) resembling north carolina macular dystrophy maps to chromosome 5. Invest Opthalmol Vis Sci 44:2178. https://doi.org/10.1167/iovs.02-1094
Rosenberg T, Roos B, Johnsen T, Bech N, Scheetz TE, Larsen M, Stone EM, Fingert JH (2010) Clinical and genetic characterization of a Danish family with North Carolina macular dystrophy. Mol Vis 16:2659–2668
Small KW, DeLuca AP, Whitmore SS, Rosenberg T, Silva-Garcia R, Udar N, Puech B, Garcia CA, Rice TA, Fishman GA, Héon E, Folk JC, Streb LM, Haas CM, Wiley LA, Scheetz TE, Fingert JH, Mullins RF, Tucker BA, Stone EM (2016) North Carolina macular dystrophy is caused by dysregulation of the retinal transcription factor PRDM13. Ophthalmology 123:9–18. https://doi.org/10.1016/j.ophtha.2015.10.006
Bowne SJ, Sullivan LS, Wheaton DK, Locke KG, Jones KD, Koboldt DC, Fulton RS, Wilson RK, Blanton SH, Birch DG, Daiger SP (2016) North Carolina macular dystrophy (MCDR1) caused by a novel tandem duplication of the PRDM13 gene. Mol Vis 22:1239–1247
Manes G, Joly W, Guignard T, Smirnov V, Berthemy S, Bocquet B, Audo I, Zeitz C, Sahel J, Cazevieille C, Sénéchal A, Deleuze JF, Blanché-Koch H, Boland A, Carroll P, Geneviève D, Zanlonghi X, Arndt C, Hamel CP, Defoort-Dhellemmes S, Meunier I (2017) A novel duplication of PRMD13 causes North Carolina macular dystrophy: overexpression of PRDM13 orthologue in drosophila eye reproduces the human phenotype. Hum Mol Genet 26:4367–4374. https://doi.org/10.1093/hmg/ddx322
Tandon M, Barnett C, Taranath D (2019) Case report: North Carolina macular dystrophy misdiagnosed as congenital ocular toxoplasmosis. Mol Vis 25:731–733
Ellingford JM, Sergouniotis PI, Jenkins E, Black GC (2017) Genome sequencing identifies a non-coding variant in the MCDR1 locus as a cause of macular dystrophy. Clin Experiment Ophthalmol 45:297–299. https://doi.org/10.1111/ceo.12825
Small KW, Tran EM, Small L, Rao RC, Shaya F (2019) Multimodal imaging and functional testing in a North Carolina macular disease family: toxoplasmosis, fovea plana, and torpedo maculopathy are phenocopies. Ophthalmology Retina 3:607–614. https://doi.org/10.1016/j.oret.2019.03.002
Bakall B, Bryan JS 3rd, Stone EM, Small KW (2018) Choroidal neovascularization in north carolina macular dystrophy responsive to anti-vascular endothelial growth factor therapy. Retin Cases Brief Rep. https://doi.org/10.1097/icb.0000000000000838
Birtel J, Gliem M, Herrmann P, Neuhaus C, Holz FG, MacLaren RE, Scholl HPN, Charbel Issa P (2021) North Carolina macular dystrophy shows a particular drusen phenotype and atrophy progression. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2021-318815
Small KW, DeLuca AP, Whitmore SS, Rosenberg T, Silva-Garcia R, Udar N, Puech B, Garcia CA, Rice TA, Fishman GA, Heon E, Folk JC, Streb LM, Haas CM, Wiley LA, Scheetz TE, Fingert JH, Mullins RF, Tucker BA, Stone EM (2016) North Carolina macular dystrophy is caused by dysregulation of the retinal transcription factor PRDM13. Ophthalmology 123:9–18. https://doi.org/10.1016/j.ophtha.2015.10.006
Cipriani V, Silva RS, Arno G, Pontikos N, Kalhoro A, Valeina S, Inashkina I, Audere M, Rutka K, Puech B, Michaelides M, van Heyningen V, Lace B, Webster AR, Moore AT (2017) Duplication events downstream of IRX1 cause North Carolina macular dystrophy at the MCDR3 locus. Sci Rep 7:7512. https://doi.org/10.1038/s41598-017-06387-6
Small KW, Garcia CA, Gallardo G, Udar N, Yelchits S (1998) North Carolina macular dystrophy (MCDR1) in Texas. Retina (Philadelphia, Pa) 18:448–452
Rabb MF, Mullen L, Yelchits S, Udar N, Small KW (1998) A North Carolina macular dystrophy phenotype in a Belizean family maps to the MCDR1 locus. Am J Ophthalmol 125:502–508. https://doi.org/10.1016/s0002-9394(99)80191-3
Pauleikhoff D, Sauer CG, Müller CR, Radermacher M, Merz A, Weber BH (1997) Clinical and genetic evidence for autosomal dominant North Carolina macular dystrophy in a German family. Am J Ophthalmol 124:412–415. https://doi.org/10.1016/s0002-9394(14)70842-6
Kim SJ, Woo SJ, Yu HG (2006) A Korean family with an early-onset autosomal dominant macular dystrophy resembling North Carolina macular dystrophy. Korean J Ophthalmol 20:220–224. https://doi.org/10.3341/kjo.2006.20.4.220
Yang Z, Tong Z, Chorich LJ, Pearson E, Yang X, Moore A, Hunt DM, Zhang K (2008) Clinical characterization and genetic mapping of North Carolina macular dystrophy. Vision Res 48:470–477. https://doi.org/10.1016/j.visres.2007.09.015
Namburi P, Khateb S, Meyer S, Bentovim T, Ratnapriya R, Khramushin A, Swaroop A, Schueler-Furman O, Banin E, Sharon D (2020) A unique PRDM13-associated variant in a Georgian Jewish family with probable North Carolina macular dystrophy and the possible contribution of a unique CFH variant. Mol Vis 26:299–310
Small KW (1998) North Carolina macular dystrophy: clinical features, genealogy, and genetic linkage analysis. Trans Am Ophthalmol Soc 96:925–961
Small KW, Killian J, McLean WC (1991) North Carolina’s dominant progressive foveal dystrophy: how progressive is it? Br J Ophthalmol 75:401–406. https://doi.org/10.1136/bjo.75.7.401
Francis PJ, Johnson S, Edmunds B, Kelsell RE, Sheridan E, Garrett C, Holder GE, Hunt DM, Moore AT (2003) Genetic linkage analysis of a novel syndrome comprising North Carolina-like macular dystrophy and progressive sensorineural hearing loss. Br J Ophthalmol 87:893–898. https://doi.org/10.1136/bjo.87.7.893
Rhee DY, Reichel E, Rogers A, Strominger M (2007) Subfoveal choroidal neovascularization in a 3-year-old child with North Carolina macular dystrophy. J AAPOS 11:614–615. https://doi.org/10.1016/j.jaapos.2007.06.010
Stone EM, Nichols BE, Kimura AE, Weingeist TA, Drack A, Sheffield VC (1994) Clinical features of a Stargardt-like dominant progressive macular dystrophy with genetic linkage to chromosome 6q. Arch Ophthalmol 112:765–772. https://doi.org/10.1001/archopht.1994.01090180063036
Khurana RN, Sun X, Pearson E, Yang Z, Harmon J, Goldberg MF, Zhang K (2009) A reappraisal of the clinical spectrum of North Carolina macular dystrophy. Ophthalmology 116:1976–1983. https://doi.org/10.1016/j.ophtha.2009.03.028
Boon CJ, Klevering BJ, Leroy BP, Hoyng CB, Keunen JE, den Hollander AI (2009) The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res 28:187–205. https://doi.org/10.1016/j.preteyeres.2009.04.002
Hartzell C, Qu Z, Putzier I, Artinian L, Chien LT, Cui Y (2005) Looking chloride channels straight in the eye: bestrophins, lipofuscinosis, and retinal degeneration. Physiology (Bethesda) 20:292–302. https://doi.org/10.1152/physiol.00021.2005
Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT (2008) Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev 88:639–672. https://doi.org/10.1152/physrev.00022.2007
Xu F, Dong F, Li H, Li X, Jiang R, Sui R (2013) Phenotypic characterization of a Chinese family with autosomal dominant cone-rod dystrophy related to GUCY2D. Doc Ophthalmol 126:233–240. https://doi.org/10.1007/s10633-013-9383-0
Fog CK, Galli GG, Lund AH (2012) PRDM proteins: important players in differentiation and disease. BioEssays 34:50–60. https://doi.org/10.1002/bies.201100107
Watanabe S, Sanuki R, Sugita Y, Imai W, Yamazaki R, Kozuka T, Ohsuga M, Furukawa T (2015) Prdm13 regulates subtype specification of retinal amacrine interneurons and modulates visual sensitivity. J Neurosci 35:8004–8020. https://doi.org/10.1523/jneurosci.0089-15.2015
Ohori S, Tsuburaya RS, Kinoshita M, Miyagi E, Mizuguchi T, Mitsuhashi S, Frith MC, Matsumoto N (2021) Long-read whole-genome sequencing identified a partial MBD5 deletion in an exome-negative patient with neurodevelopmental disorder. J Hum Genet. https://doi.org/10.1038/s10038-020-00893-8
Jiang F, Lyu GZ, Zhang VW, Li DZ (2021) Identification of thalassemia gene cluster deletion by long-read whole-genome sequencing (LR-WGS). Int J Lab Hematol. https://doi.org/10.1111/ijlh.13452
Logsdon GA, Vollger MR, Eichler EE (2020) Long-read human genome sequencing and its applications. Nat Rev Genet 21:597–614. https://doi.org/10.1038/s41576-020-0236-x
Marshall CR, Bick D, Belmont JW, Taylor SL, Ashley E, Dimmock D, Jobanputra V, Kearney HM, Kulkarni S, Rehm H (2020) The Medical Genome Initiative: moving whole-genome sequencing for rare disease diagnosis to the clinic. Genome Med 12:48. https://doi.org/10.1186/s13073-020-00748-z
Acknowledgements
The authors thank the patients for taking part in this research.
Funding
This work was supported by the National Natural Science Foundation of China (81873687).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital.
Consent for publication
Obtained.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The authors alone are responsible for the content and writing of the article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, S., Yuan, Z., Sun, Z. et al. A novel tandem duplication of PRDM13 in a Chinese family with North Carolina macular dystrophy. Graefes Arch Clin Exp Ophthalmol 260, 645–653 (2022). https://doi.org/10.1007/s00417-021-05376-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05376-w