Abstract
Purpose
To characterise longitudinal structural and functional changes in albino Sprague-Dawley rats following circumlimbal suture ocular hypertension (OHT) induction.
Methods
Ten-week-old rats (n = 24) underwent suture implantation around the limbal region in both eyes. On the next day, the suture was removed from one eye (control eyes) and left intact in the other eye (OHT eyes) of each animal. Intraocular pressure (IOP) was monitored weekly twice for the next 15 weeks. Optical coherence tomography (OCT) and electroretinogram (ERG) were measured at baseline and weeks 4, 8, 12, and 15, and eyes were then collected for histological assessment.
Results
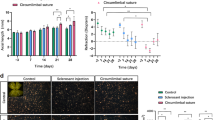
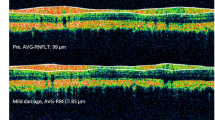
Sutured eyes (n = 12) developed IOP elevation of ~ 50% in the first 2 weeks that was sustained at ~ 25% above the control eye up to week 15 (p = 0.001). Animals with insufficient IOP elevation (n = 6), corneal changes (n = 3), and attrition (n = 3) were excluded from the analysis. OHT eyes developed significant retinal nerve fibre layer (RNFL) thinning (week 4: − 19 ± 14%, p = 0.10; week 8: − 17 ± 12%, p = 0.04; week 12: − 16 ± 10%, p = 0.04, relative to baseline) and reduction in retinal ganglion cell (RGC) density (− 32 ± 26%, p = 0.02). At week 15, both inner (9 ± 7%, p = 0.01) and outer retinal layer thicknesses (6.0 ± 5%, p = 0.001) showed a mild increase in thicknesses. The positive scotopic threshold response (− 28 ± 25%, p = 0.04) and a-wave were significantly reduced at week 12 (− 35 ± 21%; p = 0.04), whereas b-wave was not significantly affected (week 12: − 18 ± 27%, p = 0.24).
Conclusion
The circumlimbal suture model produced a chronic, moderate IOP elevation in an albino strain that led to RNFL thinning and reduced RGC density along with the reductions in ganglion and photoreceptoral cell functions. There was a small thickening in both outer and inner retinal layers.









Similar content being viewed by others
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11):2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90(3):262–267. https://doi.org/10.1136/bjo.2005.081224
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial G (2002) Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol 120(10):1268–1279. https://doi.org/10.1001/archopht.120.10.1268
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, Wilson MR, Gordon MO (2002) The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120(6):701–713
Group CN-TGS (1998) Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126(4):487–497
Bouhenni R, Dunmire J, Sewell A, Edward DP (2012) Animal models of glaucoma. Biomed Res Int 2012:692609. https://doi.org/10.1155/2012/692609
Johnson TV, Tomarev SI (2010) Rodent models of glaucoma. Brain Res Bull 81(2–3):349–358
Vecino E, Sharma SC (2011) Glaucoma animal models. In: Glaucoma-Basic and Clinical Concepts. IntechOpen, pp 319–334
Urcola JH, Hernández M, Vecino E (2006) Three experimental glaucoma models in rats: comparison of the effects of intraocular pressure elevation on retinal ganglion cell size and death. Exp Eye Res 83(2):429–437
Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA (2010) Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res 91(3):415–424
Sappington RM, Carlson BJ, Crish SD, Calkins DJ (2010) The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci 51(1):207–216
Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME (2002) Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci 43(2):402–410
Biermann J, van Oterendorp C, Stoykow C, Volz C, Jehle T, Boehringer D, Lagrèze WA (2012) Evaluation of intraocular pressure elevation in a modified laser-induced glaucoma rat model. Exp Eye Res 104:7–14
Ueda J, Sawaguchi S, Hanyu T, Yaoeda K, Fukuchi T, Abe H, Ozawa H (1998) Experimental glaucoma model in the rat induced by laser trabecular photocoagulation after an intracameral injection of India ink. Jpn J Ophthalmol 42(5):337–344
WoldeMussie E, Ruiz G, Wijono M, Wheeler LA (2001) Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci 42(12):2849–2855
Li RS, Tay DK, Chan HH, So KF (2006) Changes of retinal functions following the induction of ocular hypertension in rats using argon laser photocoagulation. Clin Exp Ophthalmol 34(6):575–583
Morrison JC, Moore C, Deppmeier LM, Gold BG, Meshul CK, Johnson EC (1997) A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res 64(1):85–96
Shareef S, Garcia-Valenzuela E, Salierno A, Walsh J, Sharma S (1995) Chronic ocular hypertension following episcleral venous occlusion in rats. Exp Eye Res 61(3):379–382
Liu H-H, Bui BV, Nguyen CT, Kezic JM, Vingrys AJ, He Z (2015) Chronic ocular hypertension induced by circumlimbal suture in rats. Invest Ophthalmol Vis Sci 56(5):2811–2820
Liu HH, He Z, Nguyen CT, Vingrys AJ, Bui BV (2017) Reversal of functional loss in a rat model of chronic intraocular pressure elevation. Ophthalmic Physiol Opt 37(1):71–81
Zhao D, Wong VHY, He Z, Nguyen CTO, Jobling AI, Fletcher E, Chinnery H, Jusuf P, Lim JK, Vingrys AJ (2018) Reversibility of retinal ganglion cell dysfunction due to chronic IOP elevation. Invest Ophthalmol Vis Sci 59(9):3696–3696
Liu H-H, Flanagan JG (2017) A mouse model of chronic ocular hypertension induced by circumlimbal suture. Invest Ophthalmol Vis Sci 58(1):353–361
Zhao D, Nguyen CT, Wong VH, Lim JK, He Z, Jobling AI, Fletcher EL, Chinnery HR, Vingrys AJ, Bui BV (2017) Characterization of the circumlimbal suture model of chronic IOP elevation in mice and assessment of changes in gene expression of stretch sensitive channels. Front Neurosci 11:41
Bayer AU, Danias J, Brodie S, Maag KP, Chen B, Shen F, Podos SM, Mittag TW (2001) Electroretinographic abnormalities in a rat glaucoma model with chronic elevated intraocular pressure. Exp Eye Res 72(6):667–677. https://doi.org/10.1006/exer.2001.1004
Ben-Shlomo G, Bakalash S, Lambrou GN, Latour E, Dawson WW, Schwartz M, Ofri R (2005) Pattern electroretinography in a rat model of ocular hypertension: functional evidence for early detection of inner retinal damage. Exp Eye Res 81(3):340–349. https://doi.org/10.1016/j.exer.2005.02.006
Kim DH, Kim HS, Ahn MD, Chun MH (2004) Ganglion cell death in rat retina by persistent intraocular pressure elevation. Korean J Ophthalmol 18(1):15–22. https://doi.org/10.3341/kjo.2004.18.1.15
Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY, So KF (2007) Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol 203(1):269–273. https://doi.org/10.1016/j.expneurol.2006.05.031
Colafrancesco V, Parisi V, Sposato V, Rossi S, Russo MA, Coassin M, Lambiase A, Aloe L (2011) Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J Glaucoma 20(2):100–108. https://doi.org/10.1097/IJG.0b013e3181d787e5
Hernández M, Urcola JH, Vecino E (2008) Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Exp Eye Res 86(5):798–806. https://doi.org/10.1016/j.exer.2008.02.008
Sarup V, McEwan GC, Thompson C, Patil KA, Sharma SC (2005) Dorzolamide and timolol saves retinal ganglion cells in glaucomatous adult rats. J Ocul Pharmacol Ther 21(6):454–462. https://doi.org/10.1089/jop.2005.21.454
Seki M, Tanaka T, Matsuda H, Togano T, Hashimoto K, Ueda J, Fukuchi T, Abe H (2005) Topically administered timolol and dorzolamide reduce intraocular pressure and protect retinal ganglion cells in a rat experimental glaucoma model. Br J Ophthalmol 89(4):504–507. https://doi.org/10.1136/bjo.2004.052860
Liu H-H, Zhang L, Shi M, Chen L, Flanagan JG (2017) Comparison of laser and circumlimbal suture induced elevation of intraocular pressure in albino CD-1 mice. PLoS One 12(11):e0189094
Lakshmanan Y, Wong FS, Yu WY, Li SZ, Choi KY, So KF, Chan HH (2019) Lycium Barbarum polysaccharides rescue neurodegeneration in an acute ocular hypertension rat model under pre- and posttreatment conditions. Invest Ophthalmol Vis Sci 60(6):2023–2033. https://doi.org/10.1167/iovs.19-26752
Bui BV, Fortune B (2004) Ganglion cell contributions to the rat full-field electroretinogram. J Physiol 555(1):153–173
Lakshmanan Y, Wong FSY, Zuo B, So K-F, Bui BV, Chan HH-L (2019) Posttreatment intervention with Lycium Barbarum polysaccharides is neuroprotective in a rat model of chronic ocular hypertension. Invest Ophthalmol Vis Sci 60(14):4606–4618. https://doi.org/10.1167/iovs.19-27886
Weitlauf C, Ward NJ, Lambert WS, Sidorova TN, Ho KW, Sappington RM, Calkins DJ (2014) Short-term increases in transient receptor potential vanilloid-1 mediate stress-induced enhancement of neuronal excitation. J Neurosci 34(46):15369–15381
Risner ML, Pasini S, Cooper ML, Lambert WS, Calkins DJ (2018) Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proc Natl Acad Sci 115(10):E2393–E2402
Choh V, Gurdita A, Tan B, Prasad RC, Bizheva K, Joos KM (2016) Short-term moderately elevated intraocular pressure is associated with elevated scotopic electroretinogram responses. Invest Ophthalmol Vis Sci 57(4):2140–2151
Mead B, Thompson A, Scheven BA, Logan A, Berry M, Leadbeater W (2014) Comparative evaluation of methods for estimating retinal ganglion cell loss in retinal sections and wholemounts. PLoS One 9(10):e110612. https://doi.org/10.1371/journal.pone.0110612
Dheer Y, Chitranshi N, Gupta V, Sharma S, Pushpitha K, Abbasi M, Mirzaei M, You Y, Graham SL, Gupta V (2019) Retinoid x receptor modulation protects against ER stress response and rescues glaucoma phenotypes in adult mice. Exp Neurol 314:111–125. https://doi.org/10.1016/j.expneurol.2019.01.015
Shindler KS, Guan Y, Ventura E, Bennett J, Rostami A (2006) Retinal ganglion cell loss induced by acute optic neuritis in a relapsing model of multiple sclerosis. Mult Scler J 12(5):526–532. https://doi.org/10.1177/1352458506070629
Horstmann L, Schmid H, Heinen AP, Kurschus FC, Dick HB, Joachim SC (2013) Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J Neuroinflammation 10:120–120. https://doi.org/10.1186/1742-2094-10-120
Renner M, Stute G, Alzureiqi M, Reinhard J, Wiemann S, Schmid H, Faissner A, Dick HB, Joachim SC (2017) Optic nerve degeneration after retinal ischemia/reperfusion in a rodent model. Front Cell Neurosci 11:254–254. https://doi.org/10.3389/fncel.2017.00254
Chaychi S, Polosa A, Lachapelle P (2015) Differences in retinal structure and function between aging male and female Sprague-Dawley rats are strongly influenced by the estrus cycle. PLoS One 10(8):e0136056
Mittag TW, Danias J, Pohorenec G, Yuan HM, Burakgazi E, Chalmers-Redman R, Podos SM, Tatton WG (2000) Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci 41(11):3451–3459
Bayer AU, Neuhardt T, May AC, Martus P, Maag K-P, Brodie S, Lütjen-Drecoll E, Podos SM, Mittag T (2001) Retinal morphology and ERG response in the DBA/2NNia mouse model of angle-closure glaucoma. Invest Ophthalmol Vis Sci 42(6):1258–1265
Lavery WJ, Muir ER, Kiel JW, Duong TQ (2012) Magnetic resonance imaging indicates decreased choroidal and retinal blood flow in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 53(2):560–564
Nork TM, Ver Hoeve JN, Poulsen GL, Nickells RW, Davis MD, Weber AJ, Sarks SH, Lemley HL, Millecchia LL (2000) Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol 118(2):235–245
Choi SS, Zawadzki RJ, Lim MC, Brandt JD, Keltner JL, Doble N, Werner JS (2011) Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol 95(1):131–141
Wilsey LJ, Reynaud J, Cull G, Burgoyne CF, Fortune B (2016) Macular structure and function in nonhuman primate experimental glaucoma. Invest Ophthalmol Vis Sci 57(4):1892–1900
Lozano DC, Twa MD (2013) Development of a rat schematic eye from in vivo biometry and the correction of lateral magnification in SD-OCT imaging. Invest Ophthalmol Vis Sci 54(9):6446–6455
Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG (2009) Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci 50(5):2328–2336
Kim EK, Park H-YL, Park CK (2017) Relationship between retinal inner nuclear layer thickness and severity of visual field loss in glaucoma. Sci Rep 7(1):5543
Choe TE, Abbott CJ, Piper C, Wang L, Fortune B (2014) Comparison of longitudinal in vivo measurements of retinal nerve fiber layer thickness and retinal ganglion cell density after optic nerve transection in rat. PLoS One 9(11):e113011. https://doi.org/10.1371/journal.pone.0113011
Bakalash S, Kipnis J, Yoles E, Schwartz M (2002) Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent. Invest Ophthalmol Vis Sci 43(8):2648–2653
Boussommier-Calleja A, Overby DR (2013) The influence of genetic background on conventional outflow facility in mice Strain-Dependent Conventional Outflow facility in mice. Invest Ophthalmol Vis Sci 54(13):8251–8258. https://doi.org/10.1167/iovs.13-13025
Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Jefferys JL, Quigley HA (2013) Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma Scleral Biomechanical Behavior in Mouse Glaucoma. Invest Ophthalmol Vis Sci 54(3):1767–1780. https://doi.org/10.1167/iovs.12-10952
Huang Y, Li Z, van Rooijen N, Wang N, Pang CP, Cui Q (2007) Different responses of macrophages in retinal ganglion cell survival after acute ocular hypertension in rats with different autoimmune backgrounds. Exp Eye Res 85(5):659–666. https://doi.org/10.1016/j.exer.2007.07.020
Dorfman AL, Polosa A, Joly S, Chemtob S, Lachapelle P (2009) Functional and structural changes resulting from strain differences in the rat model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 50(5):2436–2450
Gurdita A, Tan B, Joos KM, Bizheva K, Choh V (2017) Pigmented and albino rats differ in their responses to moderate, acute and reversible intraocular pressure elevation. Doc Ophthalmol 134(3):205–219. https://doi.org/10.1007/s10633-017-9586-x
Polosa A, Bessaklia H, Lachapelle P (2016) Strain differences in light-induced retinopathy. PLoS One 11(6):e0158082
Safa R, Osborne NN (2000) Retinas from albino rats are more susceptible to ischaemic damage than age-matched pigmented animals. Brain Res 862(1–2):36–42
Livne-Bar I, Wei J, Liu H-H, Alqawlaq S, Won G-J, Tuccitto A, Gronert K, Flanagan JG, Sivak JM (2017) Astrocyte-derived lipoxins A 4 and B 4 promote neuroprotection from acute and chronic injury. J Clin Invest 127(12):4403–4414
Acknowledgements
The authors would like to thank Dr. Hsin-Hua Liu, Centre for Eye Research, Australia, for sharing surgical technic nuances on model induction. The authors acknowledge Dr. Ricky Wing Kei WU, School of Medical and Health Sciences, Tung Wah College, Hong Kong, for availing their laboratory facilities to perform the histological procedures. The authors also thank the University Research Facilities in Behavioral and Systems Neuroscience (UBSN) and in Life Sciences (ULS), The Hong Kong Polytechnic University, for technical and facility supports.
Funding
The study is supported by the General Research Fund (PolyU 151001/17M) from Research Grants Council, HKSAR; Central Research Grant (for Research Student); and Internal Research Grants (G-YBGC, Z0GF) from The Hong Kong Polytechnic University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3240 kb).
Rights and permissions
About this article
Cite this article
Lakshmanan, Y., Wong, F.S.Y., Zuo, B. et al. Longitudinal outcomes of circumlimbal suture model-induced chronic ocular hypertension in Sprague-Dawley albino rats. Graefes Arch Clin Exp Ophthalmol 258, 2715–2728 (2020). https://doi.org/10.1007/s00417-020-04820-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04820-7