Abstract
Purpose
To study the pupillary system by combining mydriasis and multifocal pupillographic objective perimetry (mfPOP). In particular, we explored how the dynamics of recovery differ for concurrently measured direct and consensual sensitivity, response delay, and signal-to-noise ratios (SNRs) for binocular mydriasis.
Methods
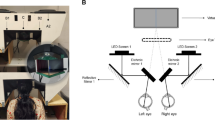
We recruited 26 normal participants, all with brown irides. The dichoptic mfPOP stimuli concurrently assessed 44-region/eye and both pupils. Two pre-dilation tests were followed by pairs of repeated tests at 1, 2, 4, 6, 8, 12, 24, and 48 h following dilation of both pupils with 1% tropicamide. Three subjects were retested with only the right pupil dilated. Linear models determined the independent effects of mydriasis upon the per-region and pupil measures over time.
Results
Post-dilation, the per-region delays initially decreased by 16.3 ± 6.02 ms (mean ± SE) (p < 0.0001, cf. baseline of 471.1 ± 4.36 ms), then increased to slower than baseline by 17.42 ± 5.57 ms after 4 h (p < 0.002), recovering to baseline at 8 h. By comparison, per-region sensitivities (constriction amplitudes) were still reduced by − 6.20 ± 0.70 μm at 8 h (p < 0.0001, cf. baseline of 21.1 ± 0.55 μm), recovered at 24 h, but rebounded at 48 h (p = 0.005). The SNRs for sensitivities and delays both recovered by 8–12 h. Across all the data, sensitivities reduced by 2.67 ± 0.25 μm/decade of age, and delay increased by 15.4 ± 1.98 ms/decade (both p < 0.00001). Data from 3 of the 26 subjects who repeated the testing for monocular dilation found that consensual response sensitivities were larger than direct for 8 h (p < 0.018).
Conclusions
The per-region sensitivities were affected for longer than SNRs or delays. Strong early SNRs indicated proportionately lower pupil noise for larger pupil diameters. Following mydriasis with tropicamide 1%, the constriction amplitude measurements with mfPOP should be considered only after 48 h, but time-to-peak can be measured after 8–12 h.







Similar content being viewed by others
References
Carle CF, James AC, Kolic M, Essex RW, Maddess T (2015) Blue multifocal pupillographic objective perimetry in glaucoma. Invest Ophthalmol Vis Sci 56:6394–6403
Sabeti F, Nolan C, Essex R, Kolic M, James AC, Maddess T (2015) Multifocal pupillography identifies changes in visual sensitivity according to severity of diabetic retinopathy in type 2 diabetes. Invest Ophthalmol Vis Sci 56:4504–4513
Sabeti F, James AC, Carle CF, Essex WR, Bell A, Maddess T (2017) Comparing multifocal pupillographic objective perimetry (mfPOP) and multifocal visual evoked potentials (mfVEP) in retinal diseases. Sci Rep 7:45847
Rosli Y, Carle CF, Ho Y, James AC, Kolic M, Rohan EMF, Maddess T (2018) Retinotopic effects of visual attention revealed by dichoptic multifocal pupillography. Sci Rep 8:2991
Ali EN, Maddess T, James AC, Voicu C, Lueck CJ (2014) Pupillary response to sparse multifocal stimuli in multiple sclerosis patients. Mult Scler J 20:854–861
Carle CF, Maddess T, James AC (2011) Contraction anisocoria: segregation, summation, and saturation in the pupillary pathway. Invest Ophthalmol Vis Sci 52(5):2365–2371
Carle CF, James AC, Rosli Y, Maddess T (2019) Localization of neuronal gain control in the pupillary response. Front Neurol 10(203):1–9
Salazar M, Shimada K, Patil PN (1976) Iris pigmentation and atropine mydriasis. J Pharmacol Exp Ther 197(1):79–88
Anicho UM, Cooper J, Feldman J, Jaanus SD, Dignam K (1999) The clinical efficacy of paremyd with and without dapiprazole in subjects with light and dark brown irides. Optom Vis Sci 76(2):94–101
Richardson RW (1982) Comparing the mydriatic effect of tropicamide with respect to iris pigmentation. J Am Optom Assoc 53(11):885–887
Cooper J, Feldman JM, Jaanus SD, Appleman W, Appel S, Horn D (1996) Pupillary dilation and funduscopy with 1.0% hydroxyamphetamine plus 0.25% tropicamide (Paremyd) versus tropicamide (0.5 or 1.0%) as a function of iris and skin pigmentation, and age. J Am Optom Assoc 67(11):669–675
Dillon JR, Tyhurst CW, Yolton RL (1977) The mydriatic effect of tropicamide on light and dark irides. J Am Optom Assoc 48(5):653–658
Becker DE (2012) Basic and clinical pharmacology of autonomic drugs. Anesth Prog 59(4):159–169
Ihekaire DE (2012) The comparative efficacy of cycloplegic drugs—tropicamide and cyclopentolate. Int J Sci Res Educ 5(3):223–246
Bradley MM, Miccoli L, Escrig MA, Lang PJ (2008) The pupil as a measure of emotional arousal and autonomic activation. Psychophysiol 45(4):602–607
Wilensky JT, Woodward HJ (1973) Acute systemic hypertension after conjunctival instillation of phenylephrine hydrochloride. Am J Ophthalmol 76(1):156–157
Drugs.com (2017) Tropicamide: FDA prescription information. www.drugs.com/pro/tropicamide.html. Accessed 15 Dec 2017
Montgomery DM, MacEwan C (1989) Pupil dilation with tropicamide. The effects of acuity, accommodation and refraction. 3(Pt 6). https://doi.org/10.1038/eye.1989.129
Maddess T, Bedford SM, Goh XL, James AC (2009) Multifocal pupillographic visual field testing in glaucoma. Clin Exp Ophthalmol 30:678–686
Carle CF, James AC, Kolic M, Essex RW, Maddess T (2014) Luminance and colour variant pupil perimetry in glaucoma. Clin Exp Ophthalmol 42(9):815–824
Carle CF, James AC, Maddess T (2013) The pupillary response to color and luminance variant multifocal stimuli. Invest Ophthalmol Vis Sci 54:467–475
Molinari JF (1983) A clinical comparison of mydriatics. J Am Optom Assoc 54(9):781–784
Ruseckaite R, Maddess T, Danta G, Lueck CJ, James AC (2005) Sparse multifocal stimuli for the detection of multiple sclerosis. Ann Neurol 57(6):904–913
James AC (2003) The pattern-pulse multifocal visual evoked potential. Invest Ophthalmol Vis Sci 44(2):879–890
James AC, Ruseckaite R, Maddess T (2005) Effect of temporal sparseness and dichoptic presentation on multifocal visual evoked potentials. Vis Neurosci 22(1):45–54
Carle CF, James AC, Kolic M, Loh YW, Maddess T (2011) High-resolution multifocal pupillographic objective perimetry in glaucoma. Invest Ophthalmol Vis Sci 52(1):604–610
Bell A, James AC, Kolic M, Essex RW, Maddess T (2010) Dichoptic multifocal pupillography reveals afferent visual field defects in early type 2 diabetes. Invest Ophthalmol Vis Sci 51(1):602–608
Cox TA, Drewes CP (1984) Contraction anisocoria resulting from half-field illumination. Am J Ophthalmol 97(5):577–582
Schmid R, Wilhelm H, Wilhelm B, Kriegbaum C, Miliczek K, Wannek U (1995) Naso-temporal differences in pupillomotor sensitivity. Invest Ophthalmol Vis Sci 37:159
Smith SA, Smith SE (1980) Contraction anisocoria: nasal versus temporal illumination. Br J Ophthalmol 64:933–934
Sutter EE, Tran D (1992) The field topography of ERG components in man—I. The photopic luminance response. Vis Res 32(3):433–446
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300(1):5–25
Usui S, Stark L (1978) Sensory and motor mechanisms interact to control amplitude of pupil noise. Vis Res 18(4):505–507
Miller D, Stark L (1964) Effect of mydriatic drugs on pupil dynamics. Res Lab Elec MIT Q Prog Rep 74:265–269
Haddad NJ, Moyer NJ, Riley FC (1970) Mydriatic effect of phenylephrine hydrochloride. Am J Ophthalmol 729–733
Aggarwal JL, Beveridge B (1971) Liberation of iris pigment in anterior chamber after instillation of 10% phenylephrine hydrochloride solution. Br J Ophthalmol 55:544–549
Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD (2005) Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433(7027):749–754
Purpura K, Tranchina D, Kaplan E, Shapley RM (1990) Light adaptation in the primate retina: analysis of changes in gain and dynamics of monkey retinal ganglion cells. Vis Neurosci 4(1):75–93
Funding
This research was supported by the Australian Research Council through the ARC Centre of Excellence in Vision Science (CE0561903), intramural funding from the ANU, and an ANU PhD scholarship to BB Rai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors FS, CFC, JvK, and TM could possibly receive royalty income from patents assigned to Konan Medical USA Inc. for a possible product based upon the mfPOP methods. The other authors declare that they have no conflict of interest.
Ethical approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Australian National University and ACT Health and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rai, B.B., Sabeti, F., Carle, C.F. et al. Recovery dynamics of multifocal pupillographic objective perimetry from tropicamide dilation. Graefes Arch Clin Exp Ophthalmol 258, 191–200 (2020). https://doi.org/10.1007/s00417-019-04523-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04523-8