Abstract
Purpose
Visual acuity (VA) is an important determinant of visual function. Here we establish procedures and recommendations for VA testing extending beyond the classical VA and thus make them available for future studies of visual function in health and disease. Specifically, we provide reference values for photopic and scotopic conventional uncrowded visual acuity (cVA) and Vernier-hyperacuity (hVA) and assess their reproducibility and dependence on contrast polarity.
Methods
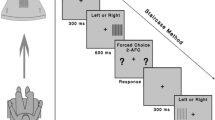
For ten observers with normal vision, we determined photopic (“p”; maximal luminance 220 cd/m2) and scotopic (“s”; maximal luminance 0.004 cd/m2; 40 min of dark adaptation) cVA and hVA, for two contrast polarities i.e. black optotypes on white background and vice versa. To assess intersession effects, two sets of measurements were obtained on different days.
Results
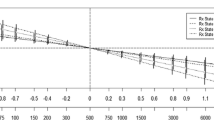
Compared to pcVA (1.32 decimal VA; − 0.12 ± 0.02 LogMAR), the phVA (14.45 decimal VA; − 1.16 ± 0.04 LogMAR) scaled (in terms of decimal visual acuity) on average with a factor 11.0, the scVA (0.12 decimal VA; 0.91 ± 0.03 LogMAR) with a factor of 0.1, and the shVA (1.47 decimal VA; − 0.17 ± 0.02 LogMAR) with a factor of 1.1. There were neither significant effects of contrast polarity (p > 0.12), nor of session (p > 0.28).
Conclusions
Our approach optimises integrated photopic and scotopic cVA and hVA measurements for general use and thus encourages the integration of these important measures of scotopic visual function in future studies. The absence of strong intersession effects demonstrates that no dedicated training session is needed to obtain scotopic and hVA measurements. The combined measures of scotopic and photopic VAs open a field of applications to study interplay and plasticity of the retinal photoreceptor systems and cortical processing in health and visual disease. As a rule of thumb, hyperacuity is 10× higher both in the photopic and scotopic range than conventional acuity. Thus, scotopic hyperacuity is close to photopic conventional acuity.


Similar content being viewed by others
Notes
In order to avoid confusion, in terms of visual acuities, we refer to ‘better’ and ‘worse’ instead of ‘higher’ and ‘lower’, as the latter terms have opposite meanings for LogMAR and decimal visual acuity.
References
Levenson JH, Kozarsky A (1990) Visual acuity. In: Walker HK, Hall WD, Hurst JW (eds) Clinical methods: the history, physical, and laboratory examinations, 3rd edn. Butterworths, Boston
Westheimer G, Bass M (2010) Visual acuity and hyperacuity//vision and vision optics, 3. ed./sponsored by the Optical Society of America. In: Bass M (ed) Handbook of optics, vol 3. McGraw-Hill, New York
Bondarko VM, Danilova MV (1997) What spatial frequency do we use to detect the orientation of a Landolt C? Vis Res 37:2153–2156. https://doi.org/10.1016/S0042-6989(97)00024-2
Livingstone MS, Hubel DH (1994) Stereopsis and positional acuity under dark adaptation. Vis Res 34:799–802. https://doi.org/10.1016/0042-6989(94)90217-8
Poggio T, Fahle M, Edelman S (1992) Fast perceptual learning in visual hyperacuity. Science 256:1018–1021
Westheimer G (1987) Visual acuity and hyperacuity: resolution, localization, form. Am J Optom Physiol Optic 64:567–574
Westheimer G, McKee PS (1977) Integration regions for visual hyperacuity. Vis Res:89–93
Duncan RO, Boynton GM (2003) Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron 38:659–671. https://doi.org/10.1016/S0896-6273(03)00265-4
Poggio T, Edelman S, Fahle M (1992) Learning of visual modules from examples. CVGIP Image Underst 56:22–30. https://doi.org/10.1016/1049-9660(92)90082-E
Levi DM, Klein SA, Aitsebaomo AP (1985) Vernier acuity, crowding and cortical magnification. Vis Res 25:963–977. https://doi.org/10.1016/0042-6989(85)90207-X
Hecht S (1928) The relation between visual acuity and illumination. J Gen Physiol 11:255–281
König A (1897) Die Abhängigkeit der Sehschärfe von der Beleuchtungsintensität. Akad Wiss Phys-Math Kl
Roelofs CO, Zeeman WPC (1919) Die Sehschärfe im Halbdunkel, zugleich ein Beitrag zur Kenntnis der Nachtblindheit. Albrecht Von Graefes Arch Für Ophthalmol 99:174–194. https://doi.org/10.1007/BF02175135
Curcio CA, Sloan KR, Kalina RE, Hendrickson AE (1990) Human photoreceptor topography. J Comp Neurol 292:497–523. https://doi.org/10.1002/cne.902920402
Osterberg G (1935) Topography of the layer of rods and cones in the human retina. Acta Ophthalmol Suppl 6:1–103
Baseler HA, Brewer AA, Sharpe LT et al (2002) Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nat Neurosci 5:364–370. https://doi.org/10.1038/nn817
Zobor D, Werner A, Stanzial F et al (2017) The clinical phenotype of cnga3-related achromatopsia: pretreatment characterization in preparation of a gene replacement therapy trial. Invest Ophthalmol Vis Sci 58:821–832. https://doi.org/10.1167/iovs.16-20427
Kampmeier J, Zorn MMC, Lang GK et al (2006) Vergleich des preferential-hyperacuity-perimeter (PHP)-tests mit dem Amsler-Netz-test bei der diagnose verschiedener Stadien der altersbezogenen Makuladegeneration. Klin Monatsblätter Für Augenheilkd 223:752–756. https://doi.org/10.1055/s-2006-926880
Loewenstein A, Malach R, Goldstein M et al (2003) Replacing the Amsler grid: a new method for monitoring patients with age-related macular degeneration. Ophthalmology 110:966–970. https://doi.org/10.1016/S0161-6420(03)00074-5
Querques G, Berboucha E, Leveziel N et al (2011) Preferential hyperacuity perimeter in assessing responsiveness to ranibizumab therapy for exudative age-related macular degeneration. Br J Ophthalmol 95:986–991. https://doi.org/10.1136/bjo.2010.190942
Yu S-Y, Kwak H-W, Kim M (2014) Association between hyperacuity defects and retinal microstructure in polypoidal choroidal vasculopathy. Indian J Ophthalmol 62:702. https://doi.org/10.4103/0301-4738.121132
Simunovic MP (2015) metamorphopsia and its quantification. Retina 35:1285. https://doi.org/10.1097/IAE.0000000000000581
Faes L, Bodmer NS, Bachmann LM et al (2014) Diagnostic accuracy of the Amsler grid and the preferential hyperacuity perimetry in the screening of patients with age-related macular degeneration: systematic review and meta-analysis. Eye 28:788–796. https://doi.org/10.1038/eye.2014.104
Group PHP (php) R (2005) Results of a multicenter clinical trial to evaluate the preferential hyperacuity perimeter for detection of age-related macular degeneration. Retina 25:296
Meier K, Giaschi D (2017) Unilateral amblyopia affects two eyes: fellow eye deficits in amblyopia. Invest Ophthalmol Vis Sci 58:1779–1800. https://doi.org/10.1167/iovs.16-20964
Dallala R, Wang Y-Z, Hess RF (2010) The global shape detection deficit in strabismic amblyopia: contribution of local orientation and position. Vis Res 50:1612–1617. https://doi.org/10.1016/j.visres.2010.05.023
Subramanian V, Morale SE, Wang Y-Z, Birch EE (2012) Abnormal radial deformation hyperacuity in children with strabismic amblyopia. Invest Ophthalmol Vis Sci 53:3303. https://doi.org/10.1167/iovs.11-8774
Watt RJ, Hess RF (1987) Spatial information and uncertainty in anisometropic amblyopia. Vis Res 27:661–674. https://doi.org/10.1016/0042-6989(87)90050-2
Bach M (2016) Manual of the Freiburg Vision Test “FrACT”, Version 3.9.8. http://docplayer.net/37653824-Manual-of-the-freiburg-vision-test-fract-version-3-9-8.html. Accessed 5 Jun 2019
Bach M, Schäfer K (2016) Visual acuity testing: feedback affects neither outcome nor reproducibility, but leaves participants happier. PLoS One 11:e0147803 https://doi.org/10.1371/journal.pone.0147803
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Martin Bland J, Altman Douglas G (1986) Statistical methods for assessing agreement between two methods of clinical. Lancet 327:307–310. https://doi.org/10.1016/S0140-6736(86)90837-8
Wilson HR (1986) Responses of spatial mechanisms can explain hyperacuity. Vis Res 26:453–469. https://doi.org/10.1016/0042-6989(86)90188-4
Crist RE, Kapadia MK, Westheimer G, Gilbert CD (1997) Perceptual learning of spatial localization: specificity for orientation, position, and context. J Neurophysiol 78:2889–2894. https://doi.org/10.1152/jn.1997.78.6.2889
Fahle M, Edelman S (1993) Long-term learning in vernier acuity: effects of stimulus orientation, range and of feedback. Vis Res 33
Shlaer S (1937) The relation between visual acuity and illumination. J Gen Physiol 21:165–188
Fahle M, Edelman S, Poggio T (1995) Fast perceptual learning in hyperacuity. Vis Res 35:3003–3013. https://doi.org/10.1016/0042-6989(95)00044-Z
Fendick M, Westheimer G (1983) Effects of practice and the separation of test targets on foveal and peripheral stereoacuity. Vis Res 23:145–1540
Mckee SP, Westheimer G (1978) Improvement in Vernier acuity with practice. Percept Psychophys 24:258–262. https://doi.org/10.3758/BF03206097
Heinrich SP, Krüger K, Bach M (2011) The dynamics of practice effects in an optotype acuity task. Graefes Arch Clin Exp Ophthalmol 249:1319–1326. https://doi.org/10.1007/s00417-011-1675-z
Petersen J (1993) Fehlerhafte Visusbestimmung und ihre quantitativen Auswirkungen. Ophthalmologe:533–538
Petersen J (1990) Zur Fehlerbreite der subjektiven Visusmessung. Ophthalmologe:604–608
Meyer CH, Lapolice DJ, Fekrat S (2005) Functional changes after photodynamic therapy with verteporfin. Am J Ophthalmol 139:214–215. https://doi.org/10.1016/j.ajo.2004.07.034
Westheimer G (2003) Visual acuity with reversed-contrast charts: I. Theoretical and psychophysical investigations. Optom Vis Sci Off Publ Am Acad Optom 80:745–748
Westheimer G, Chu P, Huang W et al (2003) Visual acuity with reversed-contrast charts: II. Clinical investigation. Optom Vis Sci Off Publ Am Acad Optom 80:749–752
Beck J, Schwartz T (1979) Venier acuity with dot test objects. Vis Res 19:313–319
Poggio T (1990) A theory of how the brain might work. Cold Spring Harb Symp Quant Biol 55:899–910. https://doi.org/10.1101/SQB.1990.055.01.084
Bach M (1996) The Freiburg visual acuity test- automatic measurement of visual acuity. Optom Vis Sci:49–53
McCulloch DL, Marmor MF, Brigell MG et al (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12. https://doi.org/10.1007/s10633-014-9473-7
Funding
This study was funded by the DFG (HO2002/12-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of the University of Magdeburg, Germany, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Suppl. Fig. 1
Relation of individual acuities (photopic and scotopic hVA, and scotopic cVA) with photopic cVA. No significant correlations between the acuities were evident. The coefficients of determination (r2) are given. Shading indicates SEMs. Note the inverted axes for the LogMAR values. (PNG 276 kb)
Rights and permissions
About this article
Cite this article
Freundlieb, P.H., Herbik, A., Kramer, F.H. et al. Determination of scotopic and photopic conventional visual acuity and hyperacuity. Graefes Arch Clin Exp Ophthalmol 258, 129–135 (2020). https://doi.org/10.1007/s00417-019-04505-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04505-w