Abstract
Purpose
The aim of this study was to identify the morphological features of the retina and choroid in Macaca fascicularis of different ages using multimodal imaging.
Methods
A total of 27 Macaca fascicularis with no ocular diseases were studied (mean age, 104.2 months; range, 1.2–223.6 months). Multimodal imaging was obtained from each subject. The morphological features were compared within four subgroups according to age.
Results
On spectrum-domain optical coherence tomography (SD-OCT), four hyper-reflective bands could be observed in the outer retina in non-infant macaques (21/21, 100%), while the interdigitation zone could not be observed in the six infant macaques. A narrow hypo-reflective band just posterior to the retinal pigment epithelium (RPE) was noted in most eyes (25/27, 92.6%). The choroidal–scleral junction (CSJ) was visible in 83.3% of infants but only in 12.5% of adults and 14.3% of the geriatric population, and it could not be seen in juveniles. There was a significant difference in CSJ visibility between the infant group and the other three groups (P < 0.001). Tessellated fundus, in which the choroidal vessels are visible through the retina, could be observed clearly with near-infrared reflectance imaging (NIR). Some granular spots were noted in juveniles, and they accumulated dramatically with age, but were absent in infants.
Conclusion
Notable morphological features can be observed in the Macaca fascicularis subjects using multimodal imaging, and these features vary distinctly according to their age. It is important to note that infant macaques had no interdigitation zone, while the other macaques had no visible CSJ but did have well-defined choroidal capillaries. Age and the features should be considered seriously in future animal studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macaca fascicularis (cynomolgus long-tailed monkey) is a cercopithecine primate that has been used extensively as an experimental animal model of ocular diseases due to its eye’s high resemblance to human eyes [1]. Many human ocular diseases, such as age-related macular degeneration (AMD), could also be observed in Macaca fascicularis [2]. Since the anatomy of the eyeball in macaques is close to that in humans, clinical imaging technologies including color fundus photography (CFP), fundus autofluorescence (FAF), fluorescein angiography (FFA), and indocyanine green angiography (ICGA) are readily adapted for primate research. Recently, multimodal imaging modalities including spectral-domain optical coherence tomography (SD-OCT) and near-infrared reflectance (NIR) imaging have been applied to address the morphological features in humans. However, very few studies have focused on the ocular morphological features of macaques, and the focus of the resultant images is generally limited to the choroid and sclera. No studies have reported on the retinal microstructures in macaques.
In this study, multimodal imaging techniques, including CFP, NIR, FFA, ICGA, and enhanced-depth imaging OCT (EDI-OCT), were performed in healthy Macaca fascicularis. The aim of this study is to describe the microstructures of the retina and choroid in Macaca fascicularis and to compare the features of macaque eyes among different age groups.
Methods
Animals
This observational study was approved by the Institutional Review Board of Zhongshan Ophthalmic Center (ZOC), Sun Yat-sen University (Guangzhou, China). The research followed the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, complied with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and was approved by the Sun Yat-sen University Institutional Animal Care and Use Committee. Twenty-seven macaques with no ocular diseases were enrolled, which were randomly selected from the macaque colony at the time of routine semiannual physical examinations. All macaques were clinically healthy during the study period. Based on the life cycle of Macaca fascicularis, all macaques were divided into four subgroups according to their age: infants (n = 6, 1–4 months), juveniles (n = 6, 4–5 years), adults (n = 8, 8–10 years), and geriatrics (n = 7, 17–18 years). In all macaques, only one eye was selected for analysis. The right eye was selected for subjects with an even-numbered birth month, while the left eye was selected for those with an odd-numbered birth month. Demographic data including age, gender, weight, and general health status were recorded for all subjects. Animal eyes that showed any retinal or choroidal lesions, or which demonstrated refractive errors ≤ − 3.0 D or ≥ 3.0 D spherical equivalent, were excluded from this study.
Multimodal imaging
All subjects underwent multimodal imaging including CFP (Smartscope M5, Optomed, Oulu, Finland), FAF, NIR imaging, SD-OCT with EDI mode, FFA, and ICGA (Heidelberg Spectralis HRA + OCT MultiColor, Heidelberg Engineering, Heidelberg, Germany). The multimodal images obtained from all subjects were evaluated. All the examinations were performed by a single researcher (P.R.). Briefly, the macaques were sedated with 10 mg kg−1 ketamine hydrochloride and 20 mg kg−1 thioethamyl intramuscularly in the morning after their usual 12-h:12-h light/dark cycle, followed by pupillary dilation with tropicamide. The head and chin rests of the imaging device were removed and replaced with a custom metal bar to allow the animals’ heads to rest comfortably. Complete ophthalmic examinations including slit-lamp biomicroscopy, dilated fundus biomicroscopy, CFP, simultaneous FFA and ICGA, and EDI-OCT were performed. Simultaneous FFA and ICGA were performed according to the standard manufacture’s protocol. Spectral domain-OCT imaging was performed using a single 30° horizontal line scan with 1536 A-scans per B-scan, centered on the fovea, in high-resolution EDI mode. Up to 100 scans (range, 50–100) were averaged for each B-scan, using the Heidelberg eye tracking Automatic Real-Time (ART) software. Near-infrared fundus autofluorescence 30° images were acquired with the HRA2 (Heidelberg Engineering) with an excitation wavelength of 787 nm. The animals were monitored by a trained technician and a veterinarian at all times.
Image analysis
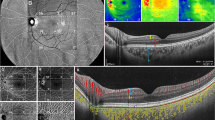
Two independent researchers (S.F. and P.R.), who are experienced in analyzing OCT images, read and analyzed all the images. First, the microstructure of the outer retina layer was reviewed. Four hyper-reflective bands, including the external limiting membrane (ELM), the ellipsoid zone (EZ), the interdigitation zone (IZ), and the RPE, were identified and defined as “absent” or “present.” Second, the visibility of choroidal capillaries (CCs) was determined. CC visibility was taken as a narrow band of hypo-reflectivity just posterior to the peak intensity of the RPE band. Third, the visibility of the choroidal–scleral junction (CSJ) was investigated. A clear CSJ is defined as an obvious dividing line between a relatively lower reflection area and a relatively higher reflection area beneath it (Fig. 1). To exclude the potential for artifacts related to the OCT beam direction causing the absence, multiple vertical and horizontal OCT sections were performed on each macaque using enhanced depth imaging (EDI) mode.
Assessment of the layer integrity 2.5 mm from the fovea was performed using the longitudinal reflectivity profile (LRP) with ImageJ software (version 1.49v; http://imagej.nih.gov/ij/). In order to compare the reflectivity profiles, the images from the left eyes were reversed. Four hyper-reflective micro-layers in the outer retina were manually identified in the resultant LRPs: the external limiting membrane (ELM), the ellipsoid zone (EZ), the interdigitation zone (IZ), and the RPE. For each eye, reflectivity values were averaged over 10 adjacent A-scans, centered at the fovea, and presented on a scale from 0 to 1.00 Gy scale units after normalization to the peak of the hyper-reflective band corresponding to the RPE, which was assigned to a value of 1.0. Reflectivity profiles from different eyes were aligned along the posterior border of this hyper-reflective RPE band for averaging.
Statistical analysis
Fisher’s exact test was used to make comparisons between groups. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics (version 22; IBM, Armonk, NY, USA).
Results
No pathological changes were observed in all subjects, and thus, multimodal images from 27 eyes were analyzed. The mean age of the animals was 104.2 months (range 1.2–223.6 months), with 14 (52%) females and 13 males (48%). The study eyes included 15 (56%) right eyes and 12 (44%) left eyes. The subjects’ demographics are given in Table 1. No remarkable features were found on CFP, FAF, FFA, and ICGA. However, there were notable features on EDI-OCT and NIR reflectance fundus photography. A representative multimodal image of Macaca fascicularis is shown in Fig. 1.
Interdigitation zone is absent in infant Macaca fascicularis according to SD-OCT
Similar with those in humans, there were four hyper-reflective bands in the outer layers of the retina on SD-OCT, which are the ELM, EZ, IZ, and RPE/Bruch’s complex. Although ELM, EZ, and RPE/Bruch’s complex were clearly demonstrated in 100% (27/27) of the subjects, IZ was observed less (21/27, 77.8%) in macaques. The subgroup analyses showed that IZ was absent in 100% (6/6) of the infant macaques (Fig. 2).
Choroidal–scleral junction in Macaca fascicularis with different ages. A distinct CSJ could be identified on EDI-OCT in infant macaques (red arrow heads) (a, animal no. 0331, 1 month old). However, the CSJ could not be visualized on EDI-OCT in the juveniles (b, animal no. 0865, 55 months old), adults (c, animal no. 5474, 126 months old), and the geriatric macaques (d, animal No. 6096, 204 months old). A clearly delineated, hypo-reflective choriocapillaris layer (b, c, and d, yellow arrow heads) could be identified. The ill-delineated CC layer was only demonstrated in some infant macaques (a, green arrow heads)
LRPs of the retina and choroid 2.5 mm from the fovea were obtained from all the images and compared (Fig. 3). The LRPs were similar in the juvenile and geriatric macaques to those in the adults (data not shown). Three peaks and a trough could be observed clearly in the outer retina by LRP in all macaques. From the retina to the choroid, the first peak for ELM, which is mild, was noted in all the groups, and the second moderate peak beneath the ELM was representative of the EZ. However, the shape of the third peak varied greatly between infant and adult macaques. Double peaks (M-pattern) according to the IZ and RPE were demonstrated in the adult groups while a single peak (N-pattern), where the IZ was absent, could be observed in infants. The visibility of the outer retina bands is shown in Table 1.
Comparison of longitudinal reflectivity profiles between infant and non-infant Macaca fascicularis. Longitudinal reflectivity profiles of the outer layer of retina and choroid at 2.5 mm from the fovea in an infant (a, animal No. 0331, 1 month old) and an adult macaque (b, animal no. 5474, 126 months old). The LRPs were similar in juvenile and geriatric macaques with those in adult ones (data not shown). There are three peaks and a subsequent trough from the ELM to the CC layer. Although similar peaks for ELM (red dot line) and EZ (green dot line) were noted in infants and adults, an M-pattern reflectance according to the presence of IZ (purple dot line in b) and RPE (yellow dot line) was demonstrated in adults; however, an N-pattern reflectance according to absence of IZ and the presence of RPE was demonstrated in infants. There was a trough representative for the CC layer (blue dot line), while in infants, the trough is shadow and narrow (blue dot line in a); in adults, the trough is relatively deeper and wider (blue dot line in b)
Visualization of choroidal capillary and the choroid–sclera junction in healthy Macaca fascicularis
The CC layer, which showed as a narrow hypo-reflective band just posterior to the peak intensity of the RPE band, was noted in 92.6% (25/27) of the eyes (Fig. 2). Subgroup analysis showed that the choriocapillaris layer was not visualized in 2 infant macaques. In LRP, beneath the RPE, there was a notch-like trough referring to the CC layer. In infants, this trough was narrow and shallow, while in non-infants it was much wider and deeper. The presence of the CC layer and the visibility of CSJ in all four groups are shown in Table 1.
Visibility of the CC layer was not significantly different from one another (P > 0.05). Notably, CSJ could be identified clearly in only 7 macaques (25.9%, 7/27). The CSJ was visible in 83.3% (5/6) of the infants, but not in any one of the juveniles (0%, 0/6), and only in 12.5% (1/8) of the adults and 14.3% (1/7) of the geriatric macaques. There was a significant difference in the CSJ visibility between infants and other three groups (P < 0.001).
Choroidal vascular networks were well-observed by NIR
NIR images were obtained from each animal. Interestingly, some granular spots, which cannot be observed in the human fundus (data not shown), emerged in juvenile macaques. Moreover, a clear tendency could be noted in that these granular spots accumulated with age, while the spots are absent in the fundus of infant macaques (Fig. 4). Simultaneous OCT scan showed that these granula corresponded to the vascular components of the choroid (Fig. 4). Meanwhile, tessellated fundus, in which the choroidal vessels are visible through the retina, could be observed in all four groups. However, these tessellated backgrounds were much paler in infant macaques than in non-infant macaques (Fig. 5). A merged infrared reflectance image from an adult macaque showed that these tessellated pattern were similar to choroidal vessels. Furthermore, simultaneous OCT scan indicated that these streaks corresponded to the choroidal vessels (Fig. 5).
Granular spots on near-infrared reflectance images in healthy Macaca fascicularis. Near-infrared reflectance images obtained from infants (a, animal no. 0326, 1 month old), juveniles (b, animal no. 1323, 54 months old), adults (c, animal no. 5866, 126 months old), and geriatrics (d, animal no .8744, 205 month old). Granular spots were absent in infants (a), shown in juveniles (b, red arrows), and were increased in adults (c) and geriatrics (d). Simultaneous OCT scan showed the granula was corresponding to the vascular components from choroid (e, arrows)
Tessellated fundus on near-infrared reflectance images in healthy Macaca fascicularis. Tessellated fundus could be observed in all four subgroups with different ages. The tessellated patterns observed in infant macaques (a) are much paler than in non-infant macaques (b). A merged infrared reflectance image from an adult macaque showed that these patterns are similar to choroidal vessels (c). Simultaneous OCT scan also indicated that these streaks were corresponding to the choroidal vessels (arrows in A and B)
Discussion
Macaque eyes are remarkably similar to that of humans in terms of the size and anatomy, allowing clinical ocular imaging technologies to be readily adapted for primate research. Moreover, macaque eyes possess a macula, which is not present in many other mammalian species such as rodents. Since Toth et al. first demonstrated the feasibility of using OCT in primates [3], SD-OCT in macaques has been widely adopted in monkey studies, for example, in models of laser-induced choroidal neovascularization [4, 5]. Therefore, it is vital to fully illustrate the fundus structures of macaques. Indeed, a comparative analysis of the choroidal morphology in macaques and humans would provide a useful basis for future translational research in macaques. Recently, Yiu et al. compared the choroidal morphology in humans and macaques and observed the ill-defined CSJ but well-delineated choriocapillaris layer in rhesus macaques [6]. These unique features were also noted in our current study with Macaca fascicularis. We suggest that the melanin pigment in the uvea may be one of the important contributors to choroidal morphology in OCT imaging. Our study provided additional evidence and, furthermore, suggested the similarity of choroidal structures between rhesus macaques and Macaca fascicularis.
In our study, we summarized the morphological features of the retina and choroid in Macaca fascicularis specimens of different ages using multimodal imaging. Although no significant features were identified using traditional tools such as CFP, FAF, FFA, or ICGA, there were some new findings using EDI-OCT and NIR imaging. Utilizing existing SD-OCT devices and a more posterior zero-delay line to attain a greater depth of field, the EDI-OCT is a powerful tool for the visualization of the retina and choroid in vivo, which has already been used widely to investigate choroidal diseases, especially in the measurement of choroidal thickness [7]. The measurement protocol is well-established in humans since the CSJ is clearly illustrated. However, in the current study, we found that the choroid revealed a very different appearance in macaques compared to that in humans. The CSJ in the majority of macaques was poorly visualized (Fig. 2b, c, and d), even using the EDI mode, due to the significant signal attenuation in the posterior choroid. A clear CSJ (Fig. 2a, red arrow heads) could be observed in 83.3% of the infant macaques, but as the age increased, the rate decreased dramatically in juveniles, adults, and geriatrics. Only two non-infant macaques, one juvenile and one geriatric, showed a distinct CSJ. Interestingly, Moreno et al. investigated the CSJ in human infants and found that the subfoveal CSJ was more easily visualized in most young-preterm and term-aged infants than in adults [8], and they suggested that this was due to less melanin in the RPE and choroid. Later, it was proven that choroidal melanocytes but not the RPE pigmentation could affect the cross-sectional visualization of choroidal structures [6]. Since the measurement of choroidal thickness is only reliable and repeatable when a clear CSJ is observed, it is critical to recognize that, to date, the measurement of choroidal thickness using EDI SD-OCT in non-infant macaques has been impractical and might be erroneous. This should be considered seriously in future animal studies with macaques.
The choroid is a highly vascular structure that supplies nourishment to RPE cells and photoreceptors. There are three vascular layers including the choriocapillaris (CC layer), medium choroidal vessel (Sattler’s layer), and large choroidal vessel layers (Haller’s layer). Some prior studies have implicated the vital role of choriocapillaris in the pathogenesis of a variety of ocular conditions, such as glaucoma, diabetic retinopathy, and age-related macular degeneration. In human beings, the CC layer and Sattler’s layer have always been considered and analyzed as a complex because current OCT technology does not allow for delineation of these layers. In the current study, different microstructures of choroidal vasculature were noted in the macaques compared with those in humans (Supplemental Fig. 1). In most of the macaques, we found that the choroid had a clearly delineated, hypo-reflective choriocapillaris layer (Fig. 2b, c, and d, yellow arrow heads). The ill-delineated CC layer, which is similar to that in humans, was only demonstrated in some infant macaques (Fig. 2a, green arrow heads). We believe that the distinct CC layer shown in Macaca fascicularis and previously reported in rhesus macaques [6] may provide a benchmark for the assessment of morphologic changes involving the choriocapillaris in various pathologic states, thus providing a better understanding of choroidal pathology.
The four hyper-reflective bands in the outer retina—the ELM, EZ, IZ, and RPE/Bruch’s membrane complex, respectively—were observed in not only healthy adult humans but also in pediatrics. It has been reported that, in humans, multiple bands in the outer retina are distinguishable at the perifovea by 32 weeks postmenstrual age, and at the fovea by 3 months postterm. The characteristic appearance of four hyper-reflective bands is evident across the foveal region by 17 months postnatal [9]. However, to date, the physiology and clinical significance of the ILM, EZ, and RPE/Bruch’s membrane complex are not well-understood and documented, and the physiological component of the hyper-reflective band between the EZ and RPE layers is still controversial [10]. It was previously referred to as either the OS tips or the Verhoeff membrane [11] and was then later defined as the photoreceptor/RPE microvilli interface by Dubis et al. [9]. Garrido et al. referred to this band as the cone outer segment tips (COST) [12]. Spaide et al. argued that this band corresponds to an ensheathment of the cone outer segments by apical processes of the retinal pigment epithelium in a structure known as the contact cylinder [13]. Interestingly, in the current study, this band (IZ) was absent in all infant macaques. To the best of our knowledge, this is the first morphological data obtained in infant macaques. We assume that the missing band is associated with the developmental process of the photoreceptor–RPE connection in macaques.
Another interesting finding in our current study is that NIR imaging could reveal the morphology of the choroidal vessels much more clearly in Macaca fascicularis than in humans (Supplemental Fig. 1). NIR imaging showed distinct morphological features and reflectance between infants and the other three groups as well as an obvious tendency that the reflectance of choroidal vascular components accumulated with age. Due to the long wavelength, NIR can penetrate through melanin and lipofuscin [14] and, therefore, enable visualization of the choroid and may also detect pathological changes despite the presence of hemorrhages or cataracts [15]. In addition, NIR minimizes pre-stimulation of the retina, avoiding the effects of absorption changes caused by photopigment bleaching. It has been suggested that Bruch’s membrane is a significant reflector of NIR light at the fundus [15]. Therefore, we speculate that the structural differences in Bruch’s membrane between humans and macaques might account for this difference. However, little evidence exists to support this hypothesis due to the lack of studies on infant macaques. Our results indicate that, in addition to its existing usage, NIR imaging could be used to monitor choroidal disease in Macaca fascicularis.
In summary, Macaca fascicularis from all age groups underwent multimodal imaging including CFP and FAF, FFA, ICGA, NIR imaging, and EDI-OCT. Our results demonstrate that the cross-sectional visualization of outer retinal microstructures, choroidal capillaries, and CSJ with EDI-OCT is very unique when compared to the documented data in humans. To the best of our knowledge, this is the first in vivo study of the retina and choroid in macaques of different ages. Compared with age-matched humans, the SCJ is not visualized, and thus, choroid thickness is not measurable in most Macaca fascicularis subjects using SD-OCT, except in infants. In addition, Macaca fascicularis infants have no IZ. We speculate that in the absence of an IZ, the infant macaques might serve as an ideal model for identifying the components and pathological significance of this microstructure in the retina, which has been suggested as vital to visual function.
References
Okamoto H, Umeda S, Nozawa T et al (2010) Comparative proteomic analyses of macular and peripheral retina of cynomolgus monkeys (Macaca fascicularis). Exp Anim 59:171–182
Umeda S, Ayyagari R, Allikmets R et al (2005) Early-onset macular degeneration with drusen in a cynomolgus monkey (Macaca fascicularis) pedigree: exclusion of 13 candidate genes and loci. Invest Ophthalmol Vis Sci 46:683–691
Toth CA, Narayan DG, Boppart SA et al (1997) A comparison of retinal morphology viewed by optical coherence tomography and by light microscopy. Arch Ophthalmol 115:1425–1428
Onami H, Nagai N, Machida S et al (2012) Reduction of laser-induced choroidal neovascularization by intravitreal vasohibin-1 in monkey eyes. Retina 32:1204–1213
Lai K, Jin C, Tu S et al (2014) Intravitreal injection of (99)Tc-MDP inhibits the development of laser-induced choroidal neovascularization in rhesus monkeys. Graefes Arch Clin Exp Ophthalmol 252:1049–1057
Yiu G, Vuong VS, Oltjen S et al (2016) Effect of uveal melanocytes on choroidal morphology in rhesus macaques and humans on enhanced-depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 57:5764–5771
Shao L, Xu L, Chen CX et al (2013) Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54:230–233
Moreno TA, O'Connell RV, Chiu SJ et al (2013) Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci 54:4140–4147
Dubis AM, Costakos DM, Subramaniam CD et al (2012) Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol 130:1291–1300
Puche N, Querques G, Benhamou N et al (2010) High-resolution spectral domain optical coherence tomography features in adult onset foveomacular vitelliform dystrophy. Br J Ophthalmol 94:1190–1196
Srinivasan VJ, Monson BK, Wojtkowski M et al (2008) Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 49:1571–1579
Garrido MG, Beck SC, Mühlfriedel R et al (2014) Towards a quantitative OCT image analysis. PLoS One 9(6):e100080
Spaide RF, Curcio CA (2011) Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina 31:1609–1619
Elsner A, Moraes L, Beausencourt E et al (2000) Scanning laser reflectometry of retinal and subretinal tissues. Opt Express 6:243–250
Fawzi AA, Nielsen JS, Mateo-Montoya A et al (2014) Multimodal imaging of white and dark without pressure fundus lesions. Retina 34:2376–2387
Funding
This study was funded by the National Key R&D Program of China (2017YFA 0104100 and 2015CB 964600) from the Ministry of Science and Technology of China and Science and Technology Program Guangdong, China (2016A020215096).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuxin Fan and Xiaoyan Ding are equal first-authors
Electronic supplementary material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fan, S., Ding, X., Rao, P. et al. Multimodal imaging of the retina and choroid in healthy Macaca fascicularis at different ages. Graefes Arch Clin Exp Ophthalmol 257, 455–463 (2019). https://doi.org/10.1007/s00417-019-04237-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04237-x