Abstract
Purpose
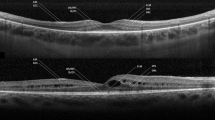
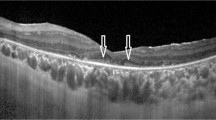
To evaluate the integrity of the outer retinal layers—outer nuclear layer (ONL), external limiting membrane (ELM), ellipsoid (EZ), and interdigitation band (IZ)—using spectral-domain optical coherence tomography and estimate their effect on visual acuity in retinitis pigmentosa (RP).
Methods
A cross-sectional study was performed in the Ophthalmology Department of Hospital de Braga, Portugal. Patients with RP followed in the Hospital de Braga during January to August 2017 were included. Exclusion criteria were lack of data, macular edema due to RP, and concomitant retinal, optic nerve, or corneal disease that could interfere with visual acuity. Age, sex, time from diagnosis, phakic status, ONL thickness, and presence or absence of foveal ELM, EZ, and IZ were correlated to the best-corrected visual acuity (BCVA).
Results
Forty-eight eyes were analyzed. There was a strong and positive correlation in BCVA between both eyes (p < .001*). ONL thickness was decreased in 95.8%. The EZ was the most absent layer (79.2%), followed by IZ (70.8%) and ELM (45.8%). A positive family history (p = .04*) and increased time from diagnosis (p = .037*) correlated with worse BCVA. A thicker ONL (p = .001*) and the presence of subfoveal ELM (p < .001*), EZ (p < .001*), and IZ (p = .02*) are correlated with better BCVA. There was a strong and positive correlation between the number of layers affected and a lower BCVA (p < .001). The presence of EZ was a significant predictor of BCVA (p = .02*).
Conclusions
The status of the outer retinal layers seems to influence BCVA. The status of the EZ was the most important predictor of BCVA but the ONL, ELM, and IZ may have a cumulative effect in the progression of visual loss.



Similar content being viewed by others
References
Puech B, Laey J-JD (2014) Retinitis pigmentosa and allied disorders. Springer Berlin Heidelberg, Berlin
Ammann F, Klein D, Franceschetti A (1965) Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J Neurol Sci 2(2):183–196
Haim M (2002) Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl 233:1–34
Boughman JA, Conneally PM, Nance WE (1980) Population genetic studies of retinitis pigmentosa. Am J Hum Genet 32(2):223–235
Berson EL (1993) Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34(5):1659–1676
Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH (1985) Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol 99(3):240–251. https://doi.org/10.1016/0002-9394(85)90351-4
Pagon RA (1988) Retinitis pigmentosa. Surv Ophthalmol 33(3):137–177
Matsuo T, Morimoto N (2007) Visual acuity and perimacular retinal layers detected by optical coherence tomography in patients with retinitis pigmentosa. Br J Ophthalmol 91(7):888–890. https://doi.org/10.1136/bjo.2007.114538
Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL (2005) The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci 46(9):3349–3354. https://doi.org/10.1167/iovs.04-1383
Ogura S, Yasukawa T, Kato A, Usui H, Hirano Y, Yoshida M, Ogura Y (2014) Wide-field fundus autofluorescence imaging to evaluate retinal function in patients with retinitis pigmentosa. Am J Ophthalmol 158(5):1093–1098
Spaide R, Curcio CA (2011) Anatomical correlates to the bands seen in the outer retina by optical coherence tomography. Retina 31(8):1609–1619
Turgut B, Demir T (2016) The new landmarks, findings and signs in optical coherence tomography. Front Ophthalmol 2(3):131–136
Mitamura Y, Mitamura-Aizawa S, Katome T, Naito T, Hagiwara A, Kumagai K, Yamamoto S (2013) Photoreceptor impairment and restoration on optical coherence tomographic image. J Ophthalmol 2013:518170. https://doi.org/10.1155/2013/518170
Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S (2009) Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye (Lond) 23(2):304–308. https://doi.org/10.1038/sj.eye.6703076
Tong KK, Lujan BJ, Zhou Y, Lin MC (2016) Directional optical coherence tomography reveals reliable outer nuclear layer measurements. Optom Vis Sci 93(7):714–719. https://doi.org/10.1097/OPX.0000000000000861
Menghini M, Lujan BJ, Zayit-Soudry S, Duncan JL (2015) Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Invest Ophthalmol Vis Sci 56(1):372–381. https://doi.org/10.1167/iovs.14-15521
Gerald A Fishman RJA, Lourenço P (1985) Prevalence of posterior subcapsular lens opacities in patients with retinitis pigmentosa. Br J Ophthalmol 69:263–266
Keane PA, Liakopoulos S, Chang KT, Wang M, Dustin L, Walsh AC, Sadda SR (2008) Relationship between optical coherence tomography retinal parameters and visual acuity in neovascular age-related macular degeneration. Ophtalmology 115(12):2206–2214
Holladay JT (1997) Proper method for calculating average visual acuity. J Refract Surg 13(4):388–391
Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M (2006) Visual acuities “hand motion” and “counting fingers” can be qualified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci 47(3):1236–1240
Chang JW, Kim JH, Kim SJ, Su YS (2014) Congenital aniridia: long-term clinical course, visual outcome and prognostic factors. Korean J Ophthalmol 28(6):479–485
Huang Q, Chen R, Lin X, Xiang Z (2017) Efficacy of carbonic anhydrase inhibitors in management of cystoid macular edema in retinitis pigmentosa: a meta-analysis. PLoS One 12(10):e0186180. https://doi.org/10.1371/journal.pone.0186180
Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL (2007) Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci 48:1298–1304
Forooghian F, Stetson PF, Meyer SA, Chew EY, Wong WT, Cukras C, Meyerle CB, Ferris FL 3rd (2010) Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina 30(1):63–70. https://doi.org/10.1097/IAE.0b013e3181bd2c5a
Alasil T, Keane PA, Updike JF, Dustin L, Ouyang Y, Walsh AC, Sadda SR (2010) Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology 117(12):2379–2386. https://doi.org/10.1016/j.ophtha.2010.03.051
Milam AH, Li ZY, Fariss RN (1998) Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 17(2):175–205
Liu G, Li H, Liu X, Xu D, Wang F (2016) Structural analysis of retinal photoreceptor ellipsoid zone and postreceptor retinal layer associated with visual acuity in patients with retinitis pigmentosa by ganglion cell analysis combined with OCT imaging. Medicine (Baltimore) 95(52):e5785. https://doi.org/10.1097/MD.0000000000005785
Holopigian K, Greenstein V, Seiple W, Carr RE (1996) Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology 103(3):398–405
Grover S, Fishman GA, Alexander KR, Anderson RJ, Derlacki DJ (1996) Visual acuity impairment in patients with retinitis pigmentosa. Ophtalmology 103(10):1593–1600
Grover S, Fishman GA, Anderson RJ, Tozatti MS, Heckenlively JR, Weleber RG, Edwards AO, Brown JJ (1999) Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophtalmology 106(9):1780–1785
Flynn MF, Fishman GA, Anderson RJ, Roberts DK (2001) Retrospective longitudinal study of visual acuity change in patients with retinitis pigmentosa. Retina 21(6):639–646
Davies EC, Pineda R (2017) Cataract surgery outcomes and complications in retinal dystrophy patients. Can J Ophthalmol 52(6):543–547
Guérin CJ, Lewis GP, Fisher SK, Anderson DH (1993) Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci 34(1):175–183
Cohen J (1997) Statistical power analysis for the behavioral sciences. Elsevier Inc.
Scott IU, PC VV, Oden NL, Ip MS, Blodi BA, Jumper JM, Figueroa M, Group SSI (2009) SCORE study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophtalmology 116(3):504–512
Hood DC, Lazow MA, Locke KG, Greenstein VC, Birch DG (2011) The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 52(1):101–108
Robson AG, Egan CA, Luong VA, Bird AC, Holder GE, Fitzke FW (2004) Comparison of fundus autofluorescence with photopic and scotopic fine-matrix mapping in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 45(11):4119–4125
Acknowledgements
The authors acknowledge the following: António Macedo (collected data); Andreia Magalhães (OCT technique supervision); Natacha Moreno, MD (collected data); Carla Ferreira, MD (collected data); Petra Gouveia, MD (writing assistance, technical editing, and proofreading); Gil Calvão-Santos, MD (proofreading and collected data); Nuno Gomes, MD (general supervision); Fernando Vaz, MD (general supervision).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were in accordance with the ethical standards of the institutional, document number 132/2017, and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All data were performed based on anonymized data and none of the presented results can identify any patient.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sousa, K., Fernandes, T., Gentil, R. et al. Outer retinal layers as predictors of visual acuity in retinitis pigmentosa: a cross-sectional study. Graefes Arch Clin Exp Ophthalmol 257, 265–271 (2019). https://doi.org/10.1007/s00417-018-4185-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4185-4