Abstract
Purpose
To evaluate the therapeutic outcome for dexamethasone implant (DEX) or intravitreal ranibizumab (IVR) injections over 6 months in patients with macular edema due to branch or central retinal vein occlusion (BRVO, CRVO), in a real-life setting.
Methods
A total of 107 patients with BRVO or CRVO were included into this retrospective single-center observational study. Patients were treated with monotherapy consisting of DEX or three monthly IVR injections following a pro re nata regimen (PRN). Best-corrected visual acuity (BCVA), central retinal thickness (CRT) and intraocular pressure (IOP) were compared between the two therapy groups after 1, 3 and 6 months.
Results
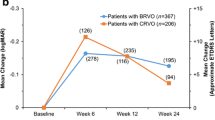
BRVO patients treated with DEX achieved a statistically significant gain in BCVA measured in logMAR after 1 month (mean gain, 95% CI: 0.21, 0.08–0.34, p = 0.001), 3 months (0.16, 0.03–0.28, p = 0.012) and 6 months (0.19, 0.07–0.32, p = 0.002), whereas patients treated with IVR showed a statistically significant BCVA gain in month 3 (mean improvement, 95% CI: 0.13, 0.01–0.26, p = 0.039) and month 6 (0.16, 0.03–0.29, p = 0.018). BCVA in CRVO patients with DEX worsened slightly at month 6 (mean worsening, 95% CI: −0.08, −0.24 to 0.08, p = 0.305), while IVR treated-patients achieved a statistically significant BCVA gain at 3 months (mean improvement, 95% CI: 0.14, 0.02–0.25, p = 0.021). Both therapies were accompanied by statistically significant CRT reductions of 150 to 200 μm (median). Adverse events reported were predictable and limited.
Conclusions
In a clinical setting, comparable improvement in BCVA and CRT were observed after DEX and IVR injections for treatment of BRVO. CRVO patients showed greater benefit with IVR.



Similar content being viewed by others
References
Haller JA, Bandello F, Belfort R, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jarques ML, Jiao J, Li XY, Whitcup SM, OZURDEX GENEVA Study Group (2010) Randomized, sham- controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 117:1134–1146
Campochiaro PA, Heier JS, Feiner L (2010) Ranibizumab for macular edema following branch retinal vein occlusion. Six-month primary end point results of a phase III study. Ophthalmology 117:1102–1112
Brown DM, Campochiaro PA, Singh RP (2010) Ranibizumab for macular edema following central retinal vein occlusion. Six-month primary end point results of a phase III study. Ophthalmology 117:1124–1133
Winterhalter S, vom Brocke GA, Klamann MK, Müller B, Joussen AM (2015) Monthly microperimetry (MP1) measurement of macular sensitivity after dexamethasone implantation (Ozurdex) in retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol 253(11):1873–1882
Mathew R, Pearce E, Muniraju R, Abdul-Hey A, Sivaprasad S (2014) Monthly OCT monitoring of Ozurdex for macular oedema related to retinal vascular diseases: re-treatment strategy (OCTOME report 1). Eye 28(3):318–326
Querques L, Querques G, Lattanzio R, Gigante SR, Del Turco C, Corradetti G, Cascavilla ML, Bandello F (2013) Repeated intravitreal dexamethasone implant (Ozurdex) for retinal vein occlusion. Ophthalmologica 229(1):21–25
Augustin AJ, Holz FG, Haritoglou C, Mayer WJ, Bopp S, Scheuerle AF, Maier M, Sekundo W, Sandner D, Shirlaw A, Hattenbach LO (2015) Retrospective, observational study in patients receiving a dexamethasone intravitreal implant 0.7mg for macular oedema secondary to retinal vein occlusion. Ophthalmologica 233:18–26. https://doi.org/10.1159/000368840
Hattenbach LO, Feltgen N, Bertelmann T et al (2017) Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B). Acta Ophthalmol. https://doi.org/10.1111/aos.13381
Hoerauf H, Feltgen N, Weiss C, Paulus EM, Schmitz-Valckenberg S, Pielen A, Puri P, Berk H, Eter N, Wiedemann P, Lang GE, Rehak M, Wolf A, Bertelmann T, Hattenbach LO, COMRADE-C Study Group (2016) Clinical efficacy and safety of Ranibizumab versus Dexamethasone for central retinal vein occlusion (COMRADE C): a European label study. Am J Opthamol. https://doi.org/10.1016/j.ajo.2016.06.016
IVAN Study Investigators, Chakravarthy U, Harding SP et al (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119:1399–1411
Lotery AJ, Gibson J, Cree AJ et al (2013) Pharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age-related macular degeneration in the IVAN study. Ophthalmology 120:2637–2643
Bach M, Kommerell G (1998) Determining visual acuity using European normal values: scientific principles and possibilities for automatic measurement. Klin Monatsbl Augenheilkd 212(4):190–195
Sugrue MF (1996) The preclinical pharmacology of dorzolamide hydrochloride, a topical carbonic anhydrase inhibitor. J Ocul Pharmacol Ther 12(3):363–376
Park HY, Lee NY, Kim JH et al (2008) Intraocular pressure lowering, change of antiapoptotic molecule expression, and neuroretinal changes by dorzolamide 2%/timolol 0.5% combination in a chronic ocular hypertension rat model. J Ocul Pharmacol Ther 24(6):563–571
Călugăru D, Călugăru M (2015) Intravitreal bevacizumab in acute central/ hemicentral retinal vein occlusions: three-year results of a prospective clinical study. J Ocul Pharmacol Ther 31(2):78–86
Mayer WJ, Haritoglou A, Wolf A et al (2015) Comparison of Intravitreal Dexamethasone implant versus Intravitreal Ranibizumab as a first-line treatment of macular Oedema due to retinal vein occlusion. Klin Monatsbl Augenheilkd 232(11):1289–1296
Chiquet C, Dupuy C, Bron AM et al (2015) Intravitreal dexamethasone implant versus anti-VEGF injection for treatment-naïve patients with retinal vein occlusion and macular edema: a 12-month follow-up study. Graefes Arch Clin Exp Ophthalmol 253(12):2095–2102
Yumusak E, Buyuktortop N, Ornek K (2016) Early results of dexamethasone implant, ranibizumab, and triamcinolone in macular edema due to branch retinal vein occlusion. Eur J Ophthalmol 26(1):54–59
Eter N, Mohr A, Wachtlin J et al (2016) Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicentre clinical trial. Graefes Arch Clin Exp Ophthalmol 255(1):77–87
Korobelnik JF, Kodjikian L, Delcour C et al (2016) Two-year, prospective, multicentre study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France. Graefes Arch Clin Exp Ophthalmol 254(12):2307–2318
Bezatis A, Spital G, Höhn F et al (2013) Functional and anatomical results after a single intravitreal Ozurdex injection in retinal vein occlusion: a 6-month follow-up – the SOLO study. Acta Ophthalmol 91(5):e340–e347
Capone A Jr, Singer MA, Dodwell DG et al (2014) Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina 34(2):342–351
Lip PL, Malick H, Damer K et al (2015) One-year outcome of bevacizumab therapy for chronic macular edema in central and branch retinal vein occlusions in real-world clinical practice in the UK. Clin Ophthalmol 25(9):1779–1784
Narayanan R, Panchal B, Das T et al (2015) A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL report no.1. Br J Ophthalmol 99(7):954–959
Larsen M, Waldstein SM, Boscia F et al (2016) Individualized Ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: twelve-month results of the CRYSTAL study. Ophthalmology 123(5):1101–1111
Farinha C, Marques JP, Almeida E et al (2015) Treatment of retinal vein occlusion with Ranibizumab in clinical practice: longer-term results and predictive factors of functional outcome. Ophthalmic Res 55(1):10–18
Campochiaro PA, Sophie R, Pearlman J et al (2014) Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology 121(1):209–219
Brynskov T, Kemp H, Sørensen TL (2014) Intravitreal ranibizumab for retinal vein occlusion through 1 year in clinical practice. Retina 34(8):1637–1643
Acknowledgements
We thank Dr. Patricia Buchholz for her advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
For this type of study, formal consent is not required.
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Winterhalter, S., Eckert, A., vom Brocke, GA. et al. Real-life clinical data for dexamethasone and ranibizumab in the treatment of branch or central retinal vein occlusion over a period of six months. Graefes Arch Clin Exp Ophthalmol 256, 267–279 (2018). https://doi.org/10.1007/s00417-017-3852-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3852-1