Abstract
Purpose
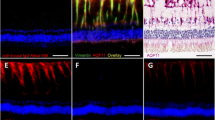
Aquaporin 4 (AQP4), a water channel protein, is known to be expressed in retinal Müller cells. The purpose of this study was to determine the effects of VEGF and AQP4 channels on the volumetric changes in Müller cells.
Methods
Retinas from diabetic rats and a cultured Müller cell line, TR-MUL5, were used in this study. Intravitreal injections of VEGF or PBS were performed on either streptozotocin (STZ)-induced diabetic or normoglycemic rats. Retinal sections were immunostained for anti-glial fibrillary acidic protein (GFAP), anti-AQP4, and anti-VEGF. VEGF protein levels from collected retinas were determined by western blot analysis. Volumetric changes and nitric oxide (NO) levels in cultured Müller cells were determined using flow cytometry (FACS), in the presence or absence of VEGF and TGN-020, a selective AQP4 inhibitor.
Results
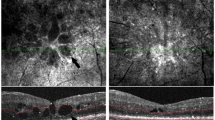
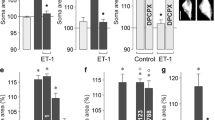
In the diabetic rat retina, VEGF immunoreactivity was concentrated in the internal retinal layers, and AQP4 immunoreactivity was higher than controls. The expressions of AQP4 were colocalized with GFAP. Protein levels of VEGF in the hyperglycemic rat retina were significantly higher than controls. FACS analyses showed that exposure to VEGF enlarged Müller cells, while exposure to TGN-020 suppressed the enlargement. Intracellular levels of NO were increased after exposure to VEGF, which was suppressed following the addition of TGN-020.
Conclusion
The observed Müller cell swelling is mediated by VEGF and AQP4.







Similar content being viewed by others
References
Whitmire W, Al-Gayyar MM, Abdelsaid M, Yousufzai BK, El-Remessy AB (2011) Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis 17:300–308
Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J, Feng Y, Hoffmann S, Gretz N, Hammes HP (2014) Systemic treatment with erythropoietin protects the neurovascular unit in a rat model of retinal neurodegeneration. PLoS One 9:e102013. doi:10.1371/journal.pone.0102013
Bandello F, Tejerina AN, Vujosevic S, Varano M, Egan C, Sivaprasad S, Menon G, Massin P, Verbraak FD, Lund-Andersen H, Martinez JP, Jurgens I, Smets RM, Coriat C, Wiedemann P, Agoas V, Querques G, Holz FG, Nunes S, Alves D, Neves C, Santos T, Ribeiro L, Cunha-Vaz J, Evicr.net (2015) Retinal layer location of increased retinal thickness in eyes with subclinical and clinical macular edema in diabetes type 2. Ophthalmic Res 54:112–117. doi:10.1159/000438792
Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34:19–48. doi:10.1016/j.preteyeres.2013.02.001
Das A, McGuire PG, Rangasamy S (2015) Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 122:1375–1394. doi:10.1016/j.ophtha.2015.03.024
Ferrara N (2016) VEGF and intraocular neovascularization: from discovery to therapy. Transl Vis Sci Technol 5:10. doi:10.1167/tvst.5.2.10
Lally DR, Shah CP, Heier JS (2016) Vascular endothelial growth factor and diabetic macular edema. Surv Ophthalmol. doi:10.1016/j.survophthal.2016.03.010
Aiello LP, Beck RW, Bressler NM, Browning DJ, Chalam KV, Davis M, Ferris FL 3rd, Glassman AR, Maturi RK, Stockdale CR, Topping TM (2011) Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology 118:e5–e14. doi:10.1016/j.ophtha.2011.09.058
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A (2006) Muller cells in the healthy and diseased retina. Prog Retin Eye Res 25:397–424. doi:10.1016/j.preteyeres.2006.05.003
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–180
Bringmann ARA, Wiedermann P (2004) Pathomechanisms of cystoid macular edema. Ophthalmic Res 36:241–249
Goodyear MJ, Crewther SG, Junghans BM (2009) A role for aquaporin-4 in fluid regulation in the inner retina. Vis Neurosci 26:159–165. doi:10.1017/s0952523809090038
Spaide RF (2016) Retinal vascular cystoid macular edema: review and new theory. Retina. doi:10.1097/iae.0000000000001158
Papadopoulos MC, Verkman AS (2007) Aquaporin-4 and brain edema. Pediatr Nephrol 22:778–784. doi:10.1007/s00467-006-0411-0
Da T, Verkman AS (2004) Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Invest Ophthalmol Vis Sci 45:4477–4483. doi:10.1167/iovs.04-0940
Wang Y, Tajkhorshid E (2010) Nitric oxide conduction by the brain aquaporin AQP4. Proteins 78:661–670. doi:10.1002/prot.22595
Gunnarson E, Zelenina M, Axehult G, Song Y, Bondar A, Krieger P, Brismar H, Zelenin S, Aperia A (2008) Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia 56:587–596. doi:10.1002/glia.20627
Plate KH, Breier G, Risau W (1994) Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol 4:207–218
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocri Rev 18:4–25. doi:10.1210/edrv.18.1.0287
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. doi:10.1371/journal.pbio.1000412
Tomi M, Funaki T, Abukawa H, Katayama K, Kondo T, Ohtsuki S, Ueda M, Obinata M, Terasaki T, Hosoya K (2003) Expression and regulation of L-cystine transporter, system xc-, in the newly developed rat retinal Muller cell line (TR-MUL). Glia 43:208–217. doi:10.1002/glia.10253
Obinata M (2007) The immortalized cell lines with differentiation potentials: their establishment and possible application. Cancer Sci 98:275–283. doi:10.1111/j.1349-7006.2007.00399.x
Vogler S, Grosche A, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A (2016) Endothelins inhibit osmotic swelling of rat retinal glial and bipolar cells by activation of growth factor signaling. Neurochem Res. doi:10.1007/s11064-016-1971-4
Hoffman RA, Johnson TS, Britt WB (1981) Flow cytometric electronic direct current volume and radiofrequency impedance measurements of single cells and particles. Cytometry 1:377–384. doi:10.1002/cyto.990010605
Potapova TA, Seidel CW, Box AC, Rancati G, Li R (2016) Transcriptome analysis of tetraploid cells identifies cyclin D2 as a facilitator of adaptation to genome doubling in the presence of p53. Mol Biol Cell 27:3065–3084. doi:10.1091/mbc.E16-05-0268
Klettner A, Westhues D, Lassen J, Bartsch S, Roider J (2013) Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: p38, nuclear factor kappaB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol Vis 19:281–291
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307. doi:10.1038/nature10144
Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E (1995) Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15:4738–4747
Vinores SA, Youssri AI, Luna JD, Chen YS, Bhargave S, Vinores MA, Schoenfeld CL, Peng B, Chan CC, LaRochelle W, Green WR, Campochiaro PA (1997) Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol 12:99–109
Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP (1996) Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103:1820–1828
Mancini JE, Ortiz G, Croxatto JO, Gallo JE (2013) Retinal upregulation of inflammatory and proangiogenic markers in a model of neonatal diabetic rats fed on a high-fat-diet. BMC Ophthalmol 13:14. doi:10.1186/1471-2415-13-14
Schey KL, Wang Z, J LW, Qi Y (2014) Aquaporins in the eye: expression, function, and roles in ocular disease. Biochim Biophys Acta 1840:1513–1523. doi:10.1016/j.bbagen.2013.10.037
Newman E, Reichenbach A (1996) The Muller cell: a functional element of the retina. Trends Neurosci 19:307–312
Qin Y, Ren H, Hoffman MR, Fan J, Zhang M, Xu G (2012) Aquaporin changes during diabetic retinopathy in rats are accelerated by systemic hypertension and are linked to the renin-angiotensin system. Invest Ophthalmol Vis Sci 53:3047–3053. doi:10.1167/iovs.11-9154
Oku H, Morishita S, Horie T, Kida T, Mimura M, Fukumoto M, Kojima S, Ikeda T (2015) Nitric oxide increases the expression of aquaporin-4 protein in rat optic nerve astrocytes through the cyclic guanosine monophosphate/protein Kinase G pathway. Ophthalmic Res 54:212–221. doi:10.1159/000440846
Kruzliak P, Novak J, Novak M (2014) Vascular endothelial growth factor inhibitor-induced hypertension: from pathophysiology to prevention and treatment based on long-acting nitric oxide donors. Am J Hypertens 27:3–13. doi:10.1093/ajh/hpt201
Shen BQ, Lee DY, Zioncheck TF (1999) Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem 274:33057–33063
Acknowledgements
The authors thank English Manuscript Editors, LLC for editorial assistance.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Grant-in-Aid for Scientific Research (C) (Researcher No. 90610105) from the Japan Society for the Promotion of Science (Tokyo, Japan), and the Osaka Eye Bank Association Fund (Osaka, Japan) provided financial support. The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval (Animal experiments)
All applicable guidelines of ARVO Statement for the care and use of animals were followed. Our experimental protocols conformed to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines.
All procedures performed in studies involving animals were in accordance with the ethical standards of the Osaka Medical College Committee on the Use and Care of Animals (Approval number: 27114) at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Kida, T., Oku, H., Horie, T. et al. Implication of VEGF and aquaporin 4 mediating Müller cell swelling to diabetic retinal edema. Graefes Arch Clin Exp Ophthalmol 255, 1149–1157 (2017). https://doi.org/10.1007/s00417-017-3631-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3631-z