Abstract
Purpose
Retinal hypoxia with consequent changes in blood flow play a role in a number of vision-threatening diseases, such as diabetic retinopathy. Previous studies have shown that the inhibition of nitric oxide synthase (NOS) and cyclooxygenase (COX) products are involved in the diameter regulation of the retinal vessels during hypoxia. Therefore, the aim of the present study was to examine the effects of an NO donor combined with COX inhibition on the diameter regulation of retinal vessels during hypoxia in normal persons.
Methods
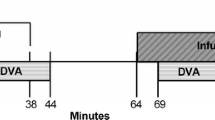
Twenty normal persons aged 21–47 years were examined. The Dynamic Vessel Analyzer (DVA) was used to measure retinal vessel diameters at rest, during isometric exercise, and during flicker stimulation. The measurements were performed during normoxia and hypoxia before and after sublingual administration of the NO donor nitroglycerin, and were repeated on a second study day after topical administration of the COX-inhibitor diclofenac.
Results
The resting diameter of arterioles and venules increased significantly during hypoxia (p < 0.0001). Hypoxia also significantly reduced the arteriolar constriction during isometric exercise, and the dilatation of the arterioles and venules during flicker stimulation (p < 0.0001). Diclofenac further reduced the arteriolar constriction induced by isometric exercise during hypoxia (p = 0.005). However, the NO-donor nitroglycerin had no effect on vascular diameters.
Conclusion
Diameter regulation of retinal vessels during hypoxia in normal persons can be influenced by the inhibition of COX products, but not by increasing the NO concentration. The findings suggest that the vasoactive effects of NO on retinal arterioles during hypoxia are saturated in normal persons.




Similar content being viewed by others
References
Bek T (2009) Inner retinal ischaemia: current understanding and needs for further investigations. Acta Ophthalmol 87(4):362–367
Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT (2004) Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol 88(7):887–891
Frederiksen CA, Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Bek T (2006) The blood pressure-induced diameter response of retinal arterioles decreases with increasing diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol 244(10):1255–1261
Koss MC (1999) Functional role of nitric oxide in regulation of ocular blood flow. Eur J Pharmacol 374(2):161–174
Schmetterer L, Polak K (2001) Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res 20(6):823–847
Petersen L, Bek T (2014) Diameter changes of retinal arterioles during acute hypoxia in vivo are modified by the inhibition of nitric oxide and prostaglandin synthesis. Curr Eye Res 40(7):737–743
Kringelholt S, Holmgaard K, Bek T (2013) Relaxation of porcine retinal arterioles during acute hypoxia in vitro depends on prostaglandin and NO synthesis in the perivascular retina. Curr Eye Res 38(9):965–971
Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L (2003) Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol 285(2):H631–H636
Santilli F, Cipollone F, Mezzetti A, Chiarelli F (2004) The role of nitric oxide in the development of diabetic angiopathy. Horm Metab Res 36(5):319–335
Frayser R, Hickam JB (1965) Effect of vasodilator drugs on the retinal blood flow in man. Arch Ophthalmol 73:640–642
Lasta M, Polak K, Luksch A, Garhofer G, Schmetterer L (2012) Effect of NO synthase inhibition on retinal vessel reaction to isometric exercise in healthy humans. Acta Ophthalmol 90(4):362–368
Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P, Kergoat H, Kotliar K, Lanzl I, Lovasik JV, Nagel E, Vilser W, Orgul S, Schmetterer L, Ocular Blood Flow Research Association (2010) Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol 88(7):717–722
Colombo E, Marconi C, Taddeo A, Cappelletti M, Villa ML, Marzorati M, Porcelli S, Vezzoli A, Della Bella S (2012) Fast reduction of peripheral blood endothelial progenitor cells in healthy humans exposed to acute systemic hypoxia. J Physiol 590(Pt 3):519–532
Ozcimen M, Sakarya Y, Goktas S, Sakarya R, Alpfidan I, Yener HI, Demir LS (2015) The effect of rebreathing and hyperventilation on retinal and choroidal vessels measured by spectral domain optical coherence tomography. Cutan Ocul Toxicol. doi:10.3109/15569527.2014.990154
Jorgensen C, Bek T (2014) Increasing oxygen saturation in larger retinal vessels after photocoagulation for diabetic retinopathy. Invest Ophthalmol Vis Sci 55(8):5365–5369
Bek T (2013) Regional morphology and pathophysiology of retinal vascular disease. Prog Retin Eye Res 36:247–259
Han TH, Qamirani E, Nelson AG, Hyduke DR, Chaudhuri G, Kuo L, Liao JC (2003) Regulation of nitric oxide consumption by hypoxic red blood cells. Proc Natl Acad Sci U S A 100(21):12504–12509
Gidday JM, Zhu Y (1995) Nitric oxide does not mediate autoregulation of retinal blood flow in newborn pig. Am J Physiol 269(3 Pt 2):H1065–H1072
Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L (2009) Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci 50(9):4029–4032
Palkovits S, Told R, Schmidl D, Boltz A, Napora KJ, Lasta M, Kaya S, Werkmeister RM, Popa-Cherecheanu A, Garhofer G, Schmetterer L (2014) Regulation of retinal oxygen metabolism in humans during graded hypoxia. Am J Physiol Heart Circ Physiol 307(10):H1412–H1418
Palkovits S, Lasta M, Told R, Schmidl D, Boltz A, Napora KJ, Werkmeister RM, Popa-Cherecheanu A, Garhofer G, Schmetterer L (2014) Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Invest Ophthalmol Vis Sci 55(8):4707–4713
Iwasaki K, Ogawa Y, Shibata S, Aoki K (2007) Acute exposure to normobaric mild hypoxia alters dynamic relationships between blood pressure and cerebral blood flow at very low frequency. J Cereb Blood Flow Metab 27(4):776–784
Willie CK, Tzeng YC, Fisher JA, Ainslie PN (2014) Integrative regulation of human brain blood flow. J Physiol 592(Pt 5):841–859
Ellis PP, Pfoff DS, Bloedow DC, Riegel M (1994) Intraocular diclofenac and flurbiprofen concentrations in human aqueous humor following topical application. J Ocul Pharmacol 10(4):677–682
Unlu N, Kocaoglan H, Sayin F, Hazirolan D, Demircan S, Basci N, Acar MA, Demir NM, Duman S (2010) Penetration of topically applied diclofenac and ketorolac into the aqueous humour and subretinal fluid: randomized clinical trial. Can J Ophthalmol 45(6):610–615
Tilma KK, Bek T (2012) Topical treatment for 1 week with latanoprost but not diclofenac reduces the diameter of dilated retinal arterioles in patients with type 1 diabetes mellitus and mild retinopathy. Acta Ophthalmol 90(8):750–755
Kringelholt S, Simonsen U, Bek T (2013) Dual effect of prostaglandins on isolated intraocular porcine ciliary arteries. Acta Ophthalmol 91(6):498–504
Hansen PO, Kringelholt S, Simonsen U, Bek T (2015) Hypoxia-induced relaxation of porcine retinal arterioles in vitro depends on inducible NO synthase and EP4 receptor stimulation in the perivascular retina. Acta Ophthalmol. doi:10.1111/aos.12669
Bek T, Hajari J, Jeppesen P (2008) Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol 246(5):763–769
Donati G, Pournaras CJ, Pizzolato GP, Tsacopoulos M (1997) Decreased nitric oxide production accounts for secondary arteriolar constriction after retinal branch vein occlusion. Invest Ophthalmol Vis Sci 38(7):1450–1457
Acknowledgments
The study was supported by Jochum and Marie Jensen’s Foundation, Synoptikfonden and the VELUX Foundation
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants, Informed consent
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Funding
The study was supported by Jochum and Marie Jensen’s Foundation, Synoptikfonden and the VELUX Foundation. The sponsors had no role in the design or conduct of this research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The authors have full control of all primary data, and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data upon request.
ClinicalTrials registration number: NCT02059018
Rights and permissions
About this article
Cite this article
Kaya, M.Y., Petersen, L. & Bek, T. Lack of effect of nitroglycerin on the diameter response of larger retinal arterioles in normal persons during hypoxia. Graefes Arch Clin Exp Ophthalmol 254, 277–283 (2016). https://doi.org/10.1007/s00417-015-3227-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3227-4