Abstract
Background
When ophthalmic drug solutions are developed and clinically applied, their influence on corneal epithelium is an important issue. In the past, cells obtained by monolayer culture in vitro were used for evaluation of such influence. We recently created an experimental model of cell damage repair closer to the live body than conventional models by using layered sheets of cultured corneal epithelium. The present study was undertaken to evaluate the influence of ophthalmic moxifloxacin hydrochloride (MFLX) solution in comparison to that of ophthalmic levofloxacin (LVFX) solution using this model.
Methods
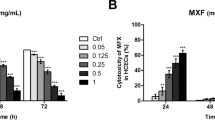
Corneal epithelium cells were collected from corneal tissue specimens of white rabbits and subjected to air-lift culture to induce layering. Epithelial cell defects were created by a sponge soaked in 1 N aqueous sodium hydroxide. After removal of the sponge, either ophthalmic MFLX solution or ophthalmic LVFX solution was dropped onto the specimens three times daily (washed 1 min after each dose, followed by continuation of air-lifting culture). The percentage of the defective area repaired (percent defect repair) was evaluated. Each of the ophthalmic MFLX solution and the ophthalmic LVFX solution was used after the stock solution was diluted fourfold (1:4). Drug-free culture medium served as the negative control. Benzalconium chloride solution (BAC) 0.01% served as the positive control.
Results
In the negative control group, complete repair of the defect with epithelial cells was seen 4 days after the start of treatment. In the positive control group, repair was suppressed. In the MFLX group and the LVFX group, the defect was repaired at each drug concentration, showing no significant difference from the negative control group. Thus, in this study using layered sheets of cultured corneal epithelium (a model closer to the living body than conventional models), the corneal epithelial defect was repaired in the ophthalmic MFLX solution treatment group and the ophthalmic LVFX solution treatment group to a degree similar to that in the negative control group.
Conclusions
These results suggest that neither MLFX nor LVFX suppresses repair of corneal epithelial damage.





Similar content being viewed by others
References
Thompson AM (2007) Ocular toxicity of fluoroquinolones. Clin Experiment Ophthalmol 35:566–577
Mallari PL, McCarty DJ, Daniell M, Taylor H (2001) Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol 131:131–133
Konishi M, Yamada M, Mashima Y (1998) Corneal ulcer associated with deposits of norfloxacin. Am J Ophthalmol 125:258–260
Castro-Munozledo F (2008) Corneal epithelial cell cultures as a tool for research, drug screening and testing. Exp Eye Res 86:459–469
Ly LT, Cavanagh HD, Petroll WM (2006) Confocal assessment of the effects of fourth-generation fluoroquinolones on the cornea. Eye Contact Lens 32:161–165
Scuderi AC, Paladino GM, Marino C, Trombetta F (2003) In vitro toxicity of netilmicin and ofloxacin on corneal epithelial cells. Cornea 22:468–472
Sakurai H, Era T, Jakt LM, Okada M, Nakai S, Nishikawa S (2006) In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells 24:575–586
Marino C, Paladino GM, Scuderi AC, Trombetta F, Mugridge K, Enea V (2005) In vivo toxicity of netilmicin and ofloxacin on intact and mechanically damaged eyes of rabbit. Cornea 24:710–716
Kim SY, Lim JA, Choi JS, Choi EC, Joo CK (2007) Comparison of antibiotic effect and corneal epithelial toxicity of levofloxacin and moxifloxacin in vitro. Cornea 26:720–725
Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ (2000) Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci 41:2506–2513
Burgalassi S, Chetoni P, Monti D, Saettone MF (2001) Cytotoxicity of potential ocular permeation enhancers evaluated on rabbit and human corneal epithelial cell lines. Toxicol Lett 122:1–8
Huhtala A, Nurmi SK, Tahti H, Salminen L, Alajuuma P, Rantala I, Helin H, Uusitalo H (2003) The immunohistochemical characterisation of an SV40-immortalised human corneal epithelial cell line. Altern Lab Anim 31:409–417
Hariprasad SM, Shah GK, Chi J, Prince RA (2005) Determination of aqueous and vitreous concentration of moxifloxacin 0.5% after delivery via a dissolvable corneal collagen shield device. J Cataract Refract Surg 31:2142–2146
Donaldson KE, Marangon FB, Schatz L, Venkatraman AS, Alfonso EC (2006) The effect of moxifloxacin on the normal human cornea. Curr Med Res Opin 22:2073–2080
Matsumoto K, Ohashi Y, Usui M, Miyanaga Y, Kitano S (2007) Phase III study of moxifloxacin ophthalmic solution in bacterial corneal infections (corneal epithelial inflammation, corneal ulcer). Stem Cells 24:575–586
Koizumi N, Kinoshita S (2003) Ocular surface reconstruction, amniotic membrane, and cultivated epithelial cells from the limbus. Br J Ophthalmol 87:1437–1439
Liu J, Song G, Wang Z, Huang B, Gao Q, Liu B, Xu Y, Liang X, Ma P, Gao N, Ge J (2007) Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp Eye Res 84:599–609
Sladowski D, Combes R, van der Valk J, Nawrot I, Gut G (2005) ESTIV questionnaire on the acquisition and use of primary human cells and tissue in toxicology. Toxicol In Vitro 19:1009–1013
Cha SH, Lee JS, Oum BS, Kim CD (2004) Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol 32:180–184
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interests in this study. The authors have full control of all primary data and they agree to allow Graefes Archive for Clinical and Experimental Ophthalmology to review their data upon request.
Rights and permissions
About this article
Cite this article
Miyake, T., Ito, N., Tajima, K. et al. Comparison of moxifloxacin and levofloxacin in an epithelial disorder model using cultured rabbit corneal epithelial cell sheets. Graefes Arch Clin Exp Ophthalmol 250, 1035–1041 (2012). https://doi.org/10.1007/s00417-011-1916-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1916-1