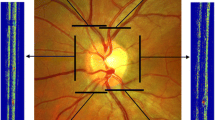
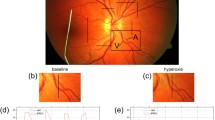
Abstract
Background
Oxygen delivery from the retinal vasculature plays a crucial role in maintaining normal retinal metabolic function. Therefore, measurements of retinal vascular oxygen tension (PO2) and PO2 longitudinal gradients (gPO2) along retinal blood vessels may help gain fundamental knowledge of retinal physiology and pathological processes.
Methods
Three-dimensional retinal vascular PO2 maps were generated in rats by optical section phosphorescence lifetime imaging. A major retinal artery and vein pair, and a smaller blood vessel (microvessel) between them were segmented, and PO2 along each blood vessel was measured. In each blood vessel, an average PO2 (mPO2) was calculated, and gPO2 was determined by linear regression analysis. Reproducibility of measurements was assessed by calculating intraclass correlation coefficient (ICC) of repeated measurements. The correlations of mPO2 and gPO2 measurements with systemic arterial oxygen tension (PaO2) and carbon dioxide tension (PaCO2) was determined.
Results
Measurements of mPO2 and gPO2 in retinal arteries, microvessels and veins were reproducible (ICC > 0.86; p < 0.01; N = 8), except for retinal arterial gPO2. Retinal arterial, microvessel and venous mPO2 were 41 ± 8, 32 ± 8 and 25 ± 7 mmHg, respectively (mean ± SD; N = 27). Retinal arterial mPO2 was correlated with PaO2 and PaCO2 (R > 0.44; p < 0.03), while retinal microvessel and venous mPO2 were only correlated with PaCO2 (R > 0.68; p < 0.01). Retinal microvessel gPO2 (−3.8 ± 1.5 mmHg/100 μm) was significantly steeper (more negative) than venous gPO2 (0.02 ± 0.43 mmHg/100 μm) (p < 0.01; N = 27), and neither were significantly correlated with PaO2 or PaCO2.
Conclusions
Quantitative measurement of mPO2 and gPO2 in the retinal microvasculature was demonstrated. A significant decrease in PO2 was observed along most retinal microvessels, indicative of substantial oxygen extraction by the retinal tissue. This method has the potential to help elucidate retinal microvascular oxygen transport in health and disease.




Similar content being viewed by others
References
Wangsa-Wirawan ND, Linsenmeier RA (2003) Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 121:547–557
Arjamaa O, Nikinmaa M (2006) Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res 83:473–483
Cringle SJ, Yu DY (2010) Oxygen supply and consumption in the retina: implications for studies of retinopathy of prematurity. Doc Ophthalmol 120:99–109
Yoneya S, Saito T, Nishiyama Y, Deguchi T, Takasu M, Gil T, Horn E (2002) Retinal oxygen saturation levels in patients with central retinal vein occlusion. Ophthalmol 109:1521–1526
Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E (2002) The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 21:359–393
Walshe TE, D’Amore PA (2008) The role of hypoxia in vascular injury and repair. Annu Rev Pathol 3:615–643
Yu DY, Cringle SJ (2001) Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 20:175–208
Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA (2007) Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol 293:H1696–H1704
Shonat RD, Kight AC (2003) Oxygen tension imaging in the mouse retina. Ann Biomed Eng 31:1084–1096
Wilson DF, Vinogradov SA, Grosul P, Vaccarezza MN, Kuroki A, Bennett J (2005) Oxygen distribution and vascular injury in the mouse eye measured by phosphorescence-lifetime imaging. Appl Opt 44:5239–5248
Shahidi M, Wanek J, Blair NP, Mori M (2009) Three-dimensional mapping of chorioretinal vascular oxygen tension in the rat. Invest Ophthalmol Vis Sci 50:820–826
Hardarson SH, Harris A, Karlsson RA, Halldorsson GH, Kagemann L, Rechtman E, Zoega GM, Eysteinsson T, Benediktsson JA, Thorsteinsson A, Jensen PK, Beach J, Stefansson E (2006) Automatic retinal oximetry. Invest Ophthalmol Vis Sci 47:5011–5016
Shakoor A, Blair NP, Mori M, Shahidi M (2006) Chorioretinal vascular oxygen tension changes in response to light flicker. Invest Ophthalmol Vis Sci 47:4962–4965
Buerk DG, Shonat RD, Riva CE, Cranstoun SD (1993) O2 gradients and countercurrent exchange in the cat vitreous humor near retinal arterioles and venules. Microvasc Res 45:134–148
Intaglietta M, Johnson PC, Winslow RM (1996) Microvascular and tissue oxygen distribution. Cardiovasc Res 32:632–643
Tsai AG, Johnson PC, Intaglietta M (2003) Oxygen gradients in the microcirculation. Physiol Rev 83:933–963
Pittman RN (2005) Oxygen transport and exchange in the microcirculation. Microcirculation 12:59–70
Torres Filho IP, Kerger H, Intaglietta M (1996) pO2 measurements in arteriolar networks. Microvasc Res 51:202–212
Vovenko E (1999) Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch 437:617–623
Stein JC, Ellsworth ML (1992) Microvascular oxygen transport: impact of a left-shifted dissociation curve. Am J Physiol Heart Circ Physiol 262:H517–H522
Ellsworth ML, Popel AS, Pittman RN (1988) Assessment and impact of heterogeneities of convective oxygen transport parameters in capillaries of striated muscle: experimental and theoretical. Microvasc Res 35:341–362
ANSI (2007) American National Standard for Safe Use of Lasers (ANSI Z136.1-2007). Laser Institute of America, Orlando
Lakowicz JR, Szmacinski H, Nowaczyk K, Berndt KW, Johnson M (1992) Fluorescence lifetime imaging. Anal Biochem 202:316–330
Shonat RD, Johnson PC (1997) Oxygen tension gradients and heterogeneity in venous microcirculation: a phosphorescence quenching study. Am J Physiol Heart Circ Physiol 272:H2233–H2240
Borgefors G (1996) On digital distance transforms in three dimensions. Comput Vis Image Underst 64:368–376
Zhou Y, Toga AW (1999) Efficient skeletonization of volumetric objects. IEEE Trans Vis Comput Graph 5:196–209
De Boor C (2001) Smoothing and least-squares approximation. In: De Boor C (ed) A practical guide to splines. Springer, New York, pp 207–210
Pannarale L, Onori P, Ripani M, Gaudio E (1996) Precapillary patterns and perivascular cells in the retinal microvasculature. A scanning electron microscope study. J Anat 188:693–703
Yamakawa K, Bhutto IA, Lu Z, Watanabe Y, Amemiya T (2001) Retinal vascular changes in rats with inherited hypercholesterolemia—corrosion cast demonstration. Curr Eye Res 22:258–265
Liu D, Wood NB, Witt N, Hughes AD, Thom SA, Xu XY (2009) Computational analysis of oxygen transport in the retinal arterial network. Curr Eye Res 34:945–956
Riva CE, Pournaras CJ, Tsacopoulos M (1986) Regulation of local oxygen tension and blood flow in the inner retina during hyperoxia. J Appl Physiol 61:592–598
Yu DY, Cringle SJ, Alder VA, Su EN (1994) Intraretinal oxygen distribution in rats as a function of systemic blood pressure. Am J Physiol 267:H2498–H2507
Pournaras CJ, Riva CE, Tsacopoulos M, Strommer K (1989) Diffusion of O2 in the retina of anesthetized miniature pigs in normoxia and hyperoxia. Exp Eye Res 49:347–360
Horvath I, Sandor NT, Ruttner Z, McLaughlin AC (1994) Role of nitric oxide in regulating cerebrocortical oxygen consumption and blood flow during hypercapnia. J Cereb Blood Flow Metab 14:503–509
Sato E, Sakamoto T, Nagaoka T, Mori F, Takakusaki K, Yoshida A (2003) Role of nitric oxide in regulation of retinal blood flow during hypercapnia in cats. Invest Ophthalmol Vis Sci 44:4947
Harris A, Arend O, Wolf S, Cantor LB, Martin BJ (1995) CO2 dependence of retinal arterial and capillary blood velocity. Acta Ophthalmol Scand 73:421–424
Venkataraman ST, Hudson C, Fisher JA, Rodrigues L, Mardimae A, Flanagan JG (2008) Retinal arteriolar and capillary vascular reactivity in response to isoxic hypercapnia. Exp Eye Res 87:535–542
Yu DY, Cringle SJ, Alder V, Su EN (1999) Intraretinal oxygen distribution in the rat with graded systemic hyperoxia and hypercapnia. Invest Ophthalmol Vis Sci 40:2082–2087
Boulpaep EL (2005) Organization of the cardiovascular system. In: Boron WF, Boulpaep EL (eds) Medical physiology. Elsevier Saunders, Philadelphia, pp 423–446
Tsacopoulos M, Baker R, David NJ, Strauss J (1973) The effect of arterial PCO2 on inner-retinal oxygen consumption rate in monkeys. Invest Ophthalmol Vis Sci 12:456–460
Acknowledgements
The authors would like to acknowledge preliminary fruitful discussions with Isa Yildirim, PhD. This study was supported by the National Eye Institute, Bethesda, MD, USA: EY17918 (MS) and EY01792 (UIC); and Research to Prevent Blindness, New York, NY, USA; senior scientific investigator award (MS) and an unrestricted departmental award.
Conflict of interest
All authors: No financial relationships with the organization that sponsored the research exists. Dr. Shahidi holds a patent for quantitative three-dimensional mapping of retinal oxygen tension technique.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the National Eye Institute, Bethesda, MD, USA: EY17918 (MS) and EY01792 (UIC); and Research to Prevent Blindness, New York, NY, USA: senior scientific investigator award (MS) and an unrestricted departmental award.
All authors have full control of all primary data, and agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review this data upon request.
Rights and permissions
About this article
Cite this article
Teng, Py., Blair, N.P., Wanek, J. et al. Oxygen tension and gradient measurements in the retinal microvasculature of rats. Graefes Arch Clin Exp Ophthalmol 250, 361–367 (2012). https://doi.org/10.1007/s00417-011-1859-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1859-6