Abstract
Purpose
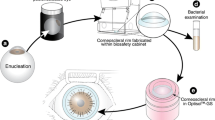
Corneas harvested post-mortem are at risk of contamination, therefore antibiotic additives are used in cold storage and organ culture systems. In the latter, sterility testing of the medium is part of the standard protocol. Intuitively, testing after longer organ culture periods should be more likely to detect contaminations than early testing, but may delay allocation. This study evaluates whether an optimal time for detection of donor cornea contamination can be identified.
Methods
The study complies with the Declaration of Helsinki. All procedures were supervised using a certified quality management system (ISO 9001:2000). Donor corneas harvested by enucleation or corneoscleral excision over 5 consecutive years were processed according to German and EC laws and guidelines. The corneas were stored in a closed organ culture system in 100 ml MEM containing penicillin, streptomycin and amphotericin B at 31°C for up to 28 days without media exchange. In 762 corneas, 10 ml samples of medium, obtained between days 3 and 8 of culture, were tested for sterility in an automated detection system (BacT/ALERT, bioMérieux). In 424 corneas, a second sterility test was performed from the same medium before release. Contamination detection probabilities were related to the culture duration before the primary test (Cochran–Armitage).
Results
Overall, 19 contaminations were found. Contaminations were bilateral in four donors. One contamination was apparent by macroscopic inspection of the medium prior to the primary sterility test; 12 were detected upon primary sterility testing. Furthermore, six primarily undetected contaminations were observed: five in the secondary sterility test and one suspected microscopically. In most cases, contamination could also be seen by medium turbidity and acidification, but in three cases macroscopic medium changes were significantly delayed or absent. No trends were found between the times of primary sterility sampling and both positive and false negative test outcome probabilities.
Conclusion
Detection probability of contaminations in organ culture media does not increase between days 3 and 8; therefore, sterility can be tested on day 3. With the recommended follow-up after sterility testing being 7 days, microbiologic release can take place after 10 days of culture. Nevertheless, the testing is not failsafe, and should always be combined with macroscopic inspection of the media.

Similar content being viewed by others
References
Albon J, Armstrong M, Tullo AB (2001) Bacterial contamination of human organ-cultured corneas. Cornea 20:260–263
Anderson RL, Vess RW, Panlilio AL, Favero MS (1990) Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone–iodine. Appl Environ Microbiol 56:3598–3600
Antonios SR, Cameron JA, Badr IA, Habash NR, Cotter JB (1991) Contamination of donor cornea: postpenetrating keratoplasty endophthalmitis. Cornea 10:217–220
Armitage WJ, Easty DL (1997) Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci 38:16–24
Borderie VM, Baudrimont M, Lopez M, Carvajal S, Laroche L (1997) Evaluation of the deswelling period in dextran-containing medium after corneal organ culture. Cornea 16:215–223
Borderie VM, Laroche L (1998) Microbiologic study of organ-cultured donor corneas. Transplantation 66:120–123
Cameron JA, Antonios SR, Cotter JB, Habash NR (1991) Endophthalmitis from contaminated donor corneas following penetrating keratoplasty. Arch Ophthalmol 109:54–59
Cheung SC, Medoff G, Schlessinger D, Kobayashi GS (1975) Stability of amphotericin B in fungal culture media. Antimicrob Agents Chemother 8:426–428
Erbezci M, Monnot PH, Michel-Briand Y, Masse M, Delbosc B (1995) Organ culture preservation of the human cornea at +31 degrees C and risk of infection. J Fr Ophtalmol 18:106–113
Everts RJ, Fowler WC, Chang DH, Reller LB (2001) Corneoscleral rim cultures: lack of utility and implications for clinical decision-making and infection prevention in the care of patients undergoing corneal transplantation. Cornea 20:586–589
Froggatt JW, Johnston JL, Galetto DW, Archer GL (1989) Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother 33:460–466
Gain P, Thuret G, Chiquet C, Vautrin AC, Carricajo A, Acquart S, Maugery J, Aubert G (2001) Use of a pair of blood culture bottles for sterility testing of corneal organ culture media. Br J Ophthalmol 85:1158–1162
Hagenah M, Bohnke M, Engelmann K, Winter R (1995) Incidence of bacterial and fungal contamination of donor corneas preserved by organ culture. Cornea 14:423–426
Jendral G, Wilhelm F, Bernhardt H, Eichler P, Kietzmann G (1999) Difficulties of fungus detection in corneal culture medium. Ophthalmologe 96:465–467
Kloess PM, Stulting RD, Waring GO III, Wilson LA (1993) Bacterial and fungal endophthalmitis after penetrating keratoplasty. Am J Ophthalmol 115:309–316
Leveille AS, McMullan FD, Cavanagh HD (1983) Endophthalmitis following penetrating keratoplasty. Ophthalmology 90:38–39
Lin CP, Bohnke M, Draeger J (1992) Effect of dextran on predamaged corneal endothelium: an organ culture study. Ophthalmic Res 24:125–128
Mahenthiralingam E, Baldwin A, Dowson CG (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104:1539–1551
Mindrup EA, Dubbel PA, Doughman DJ (1993) Betadine decontamination of donor globes. Cornea 12:324–329
Pels E, Vrensen GF (1999) Microbial decontamination of human donor eyes with povidone-iodine: penetration, toxicity, and effectiveness. Br J Ophthalmol 83:1019–1026
Pels L (1997) Organ culture: the method of choice for preservation of human donor corneas. Br J Ophthalmol 81:523–525
Redbrake C, Salla S, Frantz A (1998) Changes in human donor corneas preserved for longer than 4 weeks. Cornea 17:62–65
Redbrake C, Salla S, Nilius R, Becker J, Reim M (1997) A histochemical study of the distribution of dextran 500 in human corneas during organ culture. Curr Eye Res 16:405–411
Riedel S, Siwek G, Beekmann SE, Richter SS, Raife T, Doern GV (2006) Comparison of the BACTEC 9240 and BacT/Alert blood culture systems for detection of bacterial contamination in platelet concentrates. J Clin Microbiol 44:2262–2264
Salla S, Redbrake C, Becker J, Reim M (1995) Remarks on the vitality of the human cornea after organ culture. Cornea 14:502–508
Spelsberg H, Reinhard T, Sengler U, Daeubener W, Sundmacher R (2002) Organ-cultured corneal grafts from septic donors: a retrospective study. Eye 16:622–627
Taban M, Behrens A, Newcomb RL, Nobe MY, McDonnell PJ (2005) Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol 123:605–609
The European Directorate for the Quality of Medicines & HealthCare (2008) Europäisches Arzneibuch. Deutscher Apotheker Verlag, Stuttgart
The European Eye Banking Association (2009) The Directory of the European Eye Banking Association. www.europeaneyebanks.com
Thuret G, Carricajo A, Chiquet C, Vautrin AC, Boureille M, Acquart S, Aubert G, Maugery J, Gain P (2003) Optimizing microbiological controls of corneal organ culture media. J Fr Ophtalmol 26:792–800
Thuret G, Carricajo A, Chiquet C, Vautrin AC, Celle N, Boureille M, Acquart S, Aubert G, Maugery J, Gain P (2002) Sensitivity and rapidity of blood culture bottles in the detection of cornea organ culture media contamination by bacteria and fungi. Br J Ophthalmol 86:1422–1427
Thuret G, Carricajo A, Vautrin AC, Raberin H, Acquart S, Garraud O, Gain P, Aubert G (2005) Efficiency of blood culture bottles for the fungal sterility testing of corneal8 organ culture media. Br J Ophthalmol 89:586–590
Thuret G, Chiquet C, Bernal F, Acquart S, Romanet JP, Mouillon M, Hegelhoffer H, Burillon C, Damour O, Maugery J, Armitage WJ, Gain P (2003) Prospective, randomized clinical and endothelial evaluation of 2 storage times for cornea donor tissue in organ culture at 31 degrees C. Arch Ophthalmol 121:442–450
Wilhelmus KR, Hassan SS (2007) The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology 114:440–445
Zanetti E, Bruni A, Mucignat G, Camposampiero D, Frigo AC, Ponzin D (2005) Bacterial contamination of human organ-cultured corneas. Cornea 24:603–607
Zhao M, Thuret G, Piselli S, Pipparelli A, Acquart S, Peoc’h M, Dumollard JM, Gain P (2008) Use of poloxamers for deswelling of organ-cultured corneas. Invest Ophthalmol Vis Sci 49:550–559
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermel, M., Salla, S., Hamsley, N. et al. Detection of contamination during organ culture of the human cornea. Graefes Arch Clin Exp Ophthalmol 248, 117–126 (2010). https://doi.org/10.1007/s00417-009-1192-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1192-5