Abstract
Background
Vascular endothelial growth factor (VEGF) antagonists are currently the therapy of choice for age-related macular degeneration. Here we compared the effects of FDA-approved Ranibizumab and off-label used Bevacizumab on RPE cells, investigating their respective uptake by RPE cells over time.
Methods
Primary porcine RPE cells were treated with Bevacizumab or Ranibizumab, respectively. Uptake of the respective VEGF-antagonists was assessed with confocal laser scanning microscopy and flow cytometry. Cell death was assessed with MTT assay and VEGF secretion was measured with ELISA.
Results
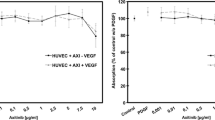
When clinical doses were applied for 1 h, Bevacizumab was taken up by RPE cells as assessed by confocal laser scanning microscopy and flow cytometry. After 24 h of incubation, and further assessed after 1d, 5d, and 7d, Bevacizumab was detected in RPE cells where it accumulated over time. The presence of Bevacizumab within RPE cells after 7d was confirmed by flow cytometry. While some Ranibizumab was found in RPE cells after 1 h of incubation when assessed with confocal laser microscopy but not by flow cytometry, no signal above control was detected after 1d, 5d, or 7d. Neither substance induced significant cell death after 7 days and no inhibitory effect on VEGF secretion was observed after day 3 of culture.
Conclusions
Bevacizumab, but not Ranibizumab, accumulates in RPE cells over time, implying substantial differences between these two drugs.







Similar content being viewed by others
References
Kim KJ, Li B, Houck K, Winer J, Ferrara N (1992) The VEGF proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors 7:53–64
Krämer I, Lipp HP (2007) Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther 32:1–14
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-VEGF antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870
Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, Ryan AM (1999) Comparison of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pharmacol 27:536–544
Heiduschka P, Fietz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, Ziemssen F, Niggemann B, Julien S, Bartz-Schmidt KU, Schraermeyer U (2007) Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci 48:2814–2823
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS (2005) Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112:1035–1047
Lynch SS, Cheng CM (2007) Bevacizumab for neovascular ocular disease. Ann Pharmacother 41:614–625
Mennel S, Callizo J, Schmidt JC, Meyer CH (2007) Acute retinal pigment epithelial tear in the untreated eye following repeated bevacizumab (Avastin) injections. Acta Ophthalmol Scand 85:689–691
Peters S, Heiduschka P, Julien S, Grisanti S, Ziemssen F, Adler M, Schraermeyer U, Bartz-Schmidt KU (2007) Ultrastructural findings in the primate eye after intravitreal injections of bevacizumab. Am J Ophthalmol 143:995–1002
European Medicines Agency, European Public Assessment Report (EPAR) on Avastin, Scientific Discussion, 2005, pp 1–61
Klettner A, Roider J (2008) Comparison of Bevacizumab, Ranibizumab and Pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci 49:4523–4527
Spitzer MS, Yoeruek E, Sierra A, Wallenfels B, Schraermeyer U, Spitzer B, Bartz-Schmidt KU, Szurman P (2007) Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol 245:1837–1842
Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ (2006) Intravitreal Bevacizumab (Avastin) for neovascular AMD. Ophthalmology 113:363–372
Gelisken F, Ziemssen F, Voelker M, Bartz-Schmidt KU (2006) Retinal pigment epithelial tear following Bevacizumab injection for neovascular age-related macular degeneration. Acta Ophthalm Scand 84:833–834
Chiang A, Chang LK, Yu F, Sarraf D (2008) Predictors of anti-VEGF associated retinal pigment epithelial tear using FA and OCT analysis. Retina 28:1265–1269
Raghavan M, Bjorkman PJ (1996) Fc Receptors and their interaction with immunoglobins. Annu Rev Cell Dev Biol 12:181–220
Eckhert CD, Hafeman DB (1986) Search for Fc and C3b receptors on black-eyed RCS rat RPE cells. Curr Eye Res 5:911–917
Elner VM, Schaffner T, Tayler K, Glagov S (1981) Immunophagocytic properties of retinal pigment epithelium cells. Science 211:74–76
Tarnowski BI, Shepherd VL, McLaughlin BJ (1988) Expression of mannose receptors for pinocytosis and phagocytosis on RPE. Invest Ophthalmol Vis Sci 29:742–748
Alge CS, Priglinger SG, Kook D, Schmid H, Haritoglou C, Welge-Lussen U, Kampik A (2006) Galectin-1 influences migration of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 47:415–426
An E, Lu X, Flippin J, Devaney JM, Halligan B, Hoffman EP, Strunnikova N, Csaky K, Hathout Y (2006) Secreted proteome profiling in human RPE cell cultures derived from donors with age-related macular degeneration and age-matched donors. J Proteome Res 5:2599–2610
Acknowledgements
The authors would like to thank A. Pfaff, S. Luick, and M. Marquardt for their excellent technical assistance and Dr. H. Oberg for his helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grants
DOG Forschungsförderung
Financial disclosure
Ranibizumab for this research was supplied by Novartis
The authors have full control of all primary data and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data if requested.
Rights and permissions
About this article
Cite this article
Klettner, A.K., Kruse, ML., Meyer, T. et al. Different properties of VEGF-antagonists: Bevacizumab but not Ranibizumab accumulates in RPE cells. Graefes Arch Clin Exp Ophthalmol 247, 1601–1608 (2009). https://doi.org/10.1007/s00417-009-1136-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1136-0