Abstract
Purpose
To investigate the response properties of the electrically evoked potentials (EEPs) elicited by intraorbital optic nerve stimulation with penetrating electrodes using different stimulus parameters.
Methods
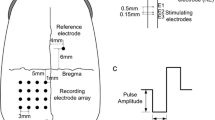
Visually evoked potentials (VEPs) were recorded as a control and for comparative purposes. Teflon-coated tungsten wire electrodes (100 μm core-diameter, 300 μm exposed tip) were inserted intraorbitally into the optic nerve. A charge-balanced biphasic current was delivered to the optic nerve via inserted wire electrodes in 26 anaesthetized rabbits. EEPs were recorded by epidural electrodes placed over the visual cortex. The charge density threshold for eliciting EEPs was determined. Stimulus pulse amplitude, duration, frequency and waveform were varied to study their effects on EEPs. After the experiments, the stimulated optic nerves were examined histologically for examination of implantation position of the stimulating electrode into the optic nerve tissue.
Results
EEPs were successfully elicited by intraorbital optic nerve stimulation with penetrating electrodes. The measured amplitude of the first large positive peak (P1) was smaller and the latency of P1 was shorter compared with VEPs. The measured charge density threshold to elicit EEPs was 21.36 ± 5.64 μC/cm2. The amplitude of P1 increased and the latency of P1 decreased with increasing pulse amplitude of fixed duration stimuli. The amplitude of P1 increased with increasing pulse duration of fixed amplitude stimuli. For fixed charge stimuli, the amplitude of P1 decreased and the latency of P1 increased as the pulse duration increased. As frequency of stimuli varied from 1 to 10 Hz, the amplitude of P1 decreased monotonically. Among the different charge-balanced biphasic pulse stimulating waveforms, the symmetrical cathode-first biphasic pulse elicited the largest amplitude of P1.
Conclusions
Our study demonstrates that intraorbital optic nerve stimulation with different stimulus parameters by penetrating electrodes can evoke cortical responses with different properties. The short-duration symmetrical cathode-first biphasic pulses of current with low frequencies are more efficacious in eliciting electrophysiological responses in the visual cortex than other stimulating waveforms.











Similar content being viewed by others
References
Branner A, Normann RA (2000) A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res Bull 51:293–306, doi:10.1016/S0361-9230(99)00231-2
Branner A, Stein RB, Normann RA (2001) Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J Neurophysiol 85:1585–1594
Chow AY, Chow VY (1997) Subretinal electrical stimulation of the rabbit retina. Neurosci Lett 225:13–16, doi:10.1016/S0304-3940(97)00185-7
Chow AY, Chow VY, Packo KH, Pollack JS, Peyman GA, Schuchard R (2004) The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol 122:460–469, doi:10.1001/archopht.122.4.460
Delbeke J, Wanet-Defalque MC, Gerard B, Troosters M, Michaux G, Veraart C (2002) The microsystems based visual prosthesis for optic nerve stimulation. Artif Organs 26:232–234, doi:10.1046/j.1525-1594.2002.06939.x
DeMarco PJ Jr, Yarbrough GL, Yee CW, McLean GY, Sagdullaev BT, Ball SL et al (2007) Stimulation via a subretinally placed prosthetic elicits central activity and induces a trophic effect on visual responses. Invest Ophthalmol Vis Sci 48:916–926, doi:10.1167/iovs.06-0811
Eckmuiller R (1997) Learning retina implants with epiretinal contacts. Ophthalmic Res 29:281–289
Fang X, Sakaguchi H, Fujikado T, Osanai M, Ikuno Y, Kamei M et al (2006) Electrophysiological and histological studies of chronically implanted intrapapillary microelectrodes in rabbit eyes. Graefes Arch Clin Exp Ophthalmol 244:364–375, doi:10.1007/s00417-005-0073-9
Fang X, Sakaguchi H, Fujikado T, Osanai M, Kanda H, Ikuno Y et al (2005) Direct stimulation of optic nerve by electrodes implanted in optic disc of rabbit eyes. Graefes Arch Clin Exp Ophthalmol 243:49–56, doi:10.1007/s00417-004-0957-0
Foerster M, Li X (1986) Evaluation of the central retina and optic nerve function in media opacities. Doc Ophthalmol 63:101–106, doi:10.1007/BF00153017
Grill WM, Mortimer JT (1995) Stimulus waveforms for selective neural stimulation. IEEE Eng Med Biol Mag 14:375–385, doi:10.1109/51.395310
Heckenlively JR, Boughman J, Friedman L (1988) Diagnosis and classification of retinitis pigmentosa. Lippincott, Philadelphia
Hornig R, Laube T, Walter P, Velikay-Parel M, Bornfeld N, Feucht M, Akguel H, Rössler G, Alteheld N, Notarp DL, Wyatt J, Richard G (2005) A method and technical equipment for an acute human trial to evaluate retinal implant technology. J Neural Eng 2:S129–S134, doi:10.1088/1741-2560/2/1/014
Humayun M (1997) Electrical stimulation of the retina in patients with photoreceptor loss. Invest Ophthalmol Vis Sci 38:S39
Humayun MS, De Juan E Jr, Weiland JD, Dagnelie G, Katona S, Greenberg R et al (1999) Pattern electrical stimulation of the human retina. Vision Res 39:2569–2576, doi:10.1016/S0042-6989(99)00052-8
Humayun MS, Prince M, De Juan E Jr, Barron Y, Moskowitz M, Klock IB et al (1999) Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci 40:143–148
Jensen RJ, Rizzo JF III (2007) Responses of ganglion cells to repetitive electrical stimulation of the retina. J Neural Eng 4:S1–S6, doi:10.1088/1741-2560/4/1/S01
Jensen RJ, Ziv OR, Rizzo JF III (2005) Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode. J Neural Eng 2:S16–S21, doi:10.1088/1741-2560/2/1/003
Jensen RJ, Ziv OR, Rizzo JF III (2005) Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Invest Ophthalmol Vis Sci 46:1486–1496, doi:10.1167/iovs.04-1018
Kanda H, Morimoto T, Fujikado T, Tano Y, Fukuda Y, Sawai H (2004) Electrophysiological studies of the feasibility of suprachoroidal-transretinal stimulation for artificial vision in normal and RCS rats. Invest Ophthalmol Vis Sci 45:560–566, doi:10.1167/iovs.02-1268
Kim SY, Sadda S, Humayun MS, De Juan E Jr, Melia BM, Green WR (2002) Morphometric analysis of the macula in eyes with geographic atrophy due to age-related macular degeneration. Retina 22:464–470, doi:10.1097/00006982-200208000-00011
Kim SY, Sadda S, Pearlman J, Humayun MS, De Juan E Jr, Melia BM et al (2002) Morphometric analysis of the macula in eyes with disciform age-related macular degeneration. Retina 22:471–477, doi:10.1097/00006982-200208000-00012
Klein R, Klein BEK, Linton KLP (1992) Prevalence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 99:933–943
Li L, Hayashida Y, Yagi T (2005) Temporal properties of retinal ganglion cell responses to local transretinal current stimuli in the frog retina. Vision Res 45:263–273, doi:10.1016/j.visres.2004.08.002
Margalit E, Maia M, Weiland JD, Greenberg RJ, Fujii GY, Torres G, Piyathaisere DV, O’Hearn TD, Liu W, Lazzi G, Dagnelie G, Scribner DA, Juan ED, Humayun MS (2002) Retinal prosthesis for the blind. Surv Ophthalmol 47:335–356, doi:10.1016/S0039-6257(02)00311-9
Maynard EM (2001) Visual prostheses. Ann Biomed Eng 3:145–168, doi:10.1146/annurev.bioeng.3.1.145
McCreery D, Agnew W, Yuen T, Bullara L (1995) Relationship between stimulus amplitude, stimulus frequency and neural damage during electrical stimulation of sciatic nerve of cat. Med Biol Eng Comput 33:426–429, doi:10.1007/BF02510526
McCreery DB, Agnew WF, Yuen TGH, Bullara L (1990) Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng 37:996–1001, doi:10.1109/10.102812
Miller RG (1997) Beyond ANOVA: Basics of Applied Statistics. Chapman & Hall, Boca Raton, FL
Ren QS, Chai XY, Wu KJ, Zhou CQ (2007) Development of C-Sight Visual Prosthesis Based on Optical Nerve Stimulation with Penetrating Electrode Array. Invest Ophthalmol Vis Sci 48:661, doi:10.1167/iovs.06-0717
Rizzo JF III, Goldbaum S, Shahin M, Denison TJ, Wyatt J (2004) In vivo electrical stimulation of rabbit retina with a microfabricated array: Strategies to maximize responses for prospective assessment of stimulus efficacy and biocompatibility. Restor Neurol Neurosci 22:429–443
Rizzo JF III, Wyatt J (1997) Prospects for a visual prosthesis. Neuroscientist 3:251–262, doi:10.1177/107385849700300413
Rizzo JF III, Wyatt J, Humayun M, De Juan E, Liu W, Chow A et al (2001) Retinal prosthesis: an encouraging first decade with major challenges ahead: editorial. Ophthalmology 108:13–14, doi:10.1016/S0161-6420(00)00430-9
Rizzo JF III, Wyatt J, Loewenstein J, Kelly S, Shire D (2003) Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci 44:5355–5361, doi:10.1167/iovs.02-0819
Rizzo JF III, Wyatt J, Loewenstein J, Kelly S, Shire D (2003) Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest Ophthalmol Vis Sci 44:5362–5369, doi:10.1167/iovs.02-0817
Rosahl SK, Mark G, Herzog M, Pantazis C, Gharabaghi F, Matthies C et al (2001) Far-field responses to stimulation of the cochlear nucleus by microsurgically placed penetrating and surface electrodes in the cat. J Neurosurg 95:845–852
Sachs HG, Schanze T, Wilms M, Rentzos A, Brunner U, Gekeler F et al (2005) Subretinal implantation and testing of polyimide film electrodes in cats. Graefes Arch Clin Exp Ophthalmol 243:464–468, doi:10.1007/s00417-004-1049-x
Sakaguchi H, Fujikado T, Kanda H, Osanai M, Fang X, Nakauchi K et al (2004) Electrical stimulation with a needle-type electrode inserted into the optic nerve in rabbit eyes. Jpn J Ophthalmol 48:552–557, doi:10.1007/s10384-004-0114-7
Santos A, Humayun MS, De Juan E Jr, Greenburg RJ, Marsh MJ, Klock IB et al (1997) Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Arch Ophthalmol 115:511–515
Shannon RV (1992) A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng 39:424–426, doi:10.1109/10.126616
Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G et al (1998) Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res 813:181–186, doi:10.1016/S0006-8993(98)00977-9
Veraart C, Wanet-Defalque MC, Gérard B, Vanlierde A, Delbeke J (2003) Pattern recognition with the optic nerve visual prosthesis. Artif Organs 27:996–1004, doi:10.1046/j.1525-1594.2003.07305.x
Walter P, Heimann K (2000) Evoked cortical potentials after electrical stimulation of the inner retina in rabbits. Graefes Arch Clin Exp Ophthalmol 238:315–318, doi:10.1007/s004170050358
Walter P, Kisvárday ZF, Görtz M, Alteheld N, Rossler G, Stieglitz T, Eysel UT (2005) Cortical activation via an implanted wireless retinal prosthesis. Invest Ophthalmol Vis Sci 46:1780–1785, doi:10.1167/iovs.04-0924
Weiland JD, Liu W, Humayun MS (2005) Retinal prosthesis. Ann Biomed Eng 7:361–401, doi:10.1146/annurev.bioeng.7.060804.100435
Zrenner E (2002) Will retinal implants restore vision? Science 295:1022–1025, doi:10.1126/science.1067996
Zrenner E, Miliczek KD, Gabel VP, Graf HG, Guenther E, Haemmerle H et al (1997) The development of subretinal microphotodiodes for replacement of degenerated photoreceptors. Ophthalmic Res 29:269–280
Zrenner E, Stett A, Weiss S, Aramant RB, Guenther E, Kohler K et al (1999) Can subretinal microphotodiodes successfully replace degenerated photoreceptors. Vision Res 39:2555–2567, doi:10.1016/S0042-6989(98)00312-5
Gekeler F, Kobuch K, Schwahn HN, Stett A, Shinoda K, Zrenner E (2004) Subretinal electrical stimulation of the rabbit retina with acutely implanted electrode arrays. Graefes Arch Clin Exp Ophthalmol 242:587–596, doi:10.1007/s00417-004-0862-6
Schwahn HN, Gekeler F, Kohler K, Kobuch K, Sachs HG, Schulmeyer F et al (2001) Studies on the feasibility of a subretinal visual prosthesis: data from Yucatan micropig and rabbit. Graefes Arch Clin Exp Ophthalmol 239:961–967, doi:10.1007/s004170100368
Humayun MS, Propst RH, de Juan EJ, McCormick K, Hickingbotham D (1994) Bipolar surface electrical stimulation of the vertebrate retina. Arch Ophthalmol 112:110–116
Humayun MS, Weilanda JD, Fujiia GY, Greenbergb R, Williamsonb R, Littleb J, Mechb B, Cimmarustib V, Boemela GV, Dagneliec G, Juan E de Jr (2003) Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res 43:2573–2581, doi:10.1016/S0042-6989(03)00457-7
Acknowledgements
The authors thank Yuxiu Liu and Xiaoliang Li for performing the animal surgery; Xiaohong Sui for providing technical supports on electrode fabrication; Niall McLoughlin for providing valuable reviews and comments on the manuscript.
This research is supported by the National Basic Research Program of China (973 Program, 2005CB724302), National Science Fund for Distinguished Young Scholars from The National Natural Science Foundation of China (60588101), Shanghai Pujiang Program (07pj14050), Shanghai Commission of Science and Technology (05DZ22318, 05DZ22325, 04DZ05114).
Author information
Authors and Affiliations
Corresponding author
Additional information
This research is supported by the National Basic Research Program of China (973 Program, 2005CB724302), National Science Fund for Distinguished Young Scholars from The National Natural Science Foundation of China (60588101), Shanghai Pujiang Program (07pj14050), Shanghai Commission of Science and Technology (05DZ22318, 05DZ22325, 04DZ05114).
Rights and permissions
About this article
Cite this article
Li, L., Cao, P., Sun, M. et al. Intraorbital optic nerve stimulation with penetrating electrodes: in vivo electrophysiology study in rabbits. Graefes Arch Clin Exp Ophthalmol 247, 349–361 (2009). https://doi.org/10.1007/s00417-008-0977-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0977-2