Abstract
Background
A wide variety of pathological pathways may result in age-related macular degeneration. Because of its complexity, there is no comprehensive model of the disease yet. One key feature is the accumulation of the autofluorescent pigment lipofuscin in the retinal pigment epithelium (RPE). Thus, we developed an organotypic perfusion culture model of the porcine ocular fundus, generating lipofuscin under exposure to blue light and hydrogen peroxide.
Methods
Porcine fundi (choroid, Bruch’s membrane, RPE, and retina) were explanted in toto, transferred into a perfusion culture chamber, perfused with cell culture medium and kept at 37°C. Free radical stress was induced by supplementation of H2O2, and/or the specimens were exposed to blue light, or kept untreated as controls. After a culture period of 7 days, the specimens were subject to microscopic inspection, histology, fluorescence microscopy, and measurement of fluorescence spectra as well as fluorescence decay times.
Results
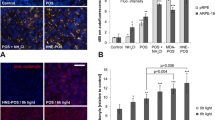
Histology showed atrophic ganglion cells and rod outer segments. All other tissue structures were morphologically intact. Compared to the controls, RPE and retina exposed to light showed increased fluorescence, which was shifted towards shorter wavelengths. The fluorescence spectra and decays resembled that of lipofuscin granules isolated from human donor eyes. HPLC analysis revealed the abundance of the lipofuscin component N-retinylidene-N-retinylethanolamine (A2E), its precursor products, as well as two new, green-emitting fluorophores.
Conclusions
Porcine ocular fundi were successfully preserved in an organotypic perfusion culture for 7 days, and exhibited remarkable autofluorescence after light and free radical exposure, making the model suitable for investigations of lipofuscinogenesis.







Similar content being viewed by others
References
Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK (2003) An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 9:1390–1397
Armstrong D, Santangelo G, Connole E (1981) The distribution of peroxide regulating enzymes in the canine eye. Curr Eye Res 1:225–242
Bindewald A, Bird AC, Dandekar SS, Dolar-Szczasny J, Dreyhaupt J, Fitzke FW, Einbock W, Holz FG, Jorzik JJ, Keilhauer C, Lois N, Mlynski J, Pauleikhoff D, Staurenghi G, Wolf S (2005) Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Ophthalmol Vis Sci 46:3309–3314
Bindewald-Wittich A, Han M, Schmitz-Valckenberg S, Snyder SR, Giese G, Bille JF, Holz FG (2006) Two-photon-excited fluorescence imaging of human RPE cells with a femtosecond Ti:Sapphire laser. Invest Ophthalmol Vis Sci 47:4553–4557
Choudhary S, Xiao T, Srivastava S, Zhang W, Chan LL, Vergara LA, Van Kuijk FJ, Ansari NH (2005) Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol 204:122–134
Cringle SJ, Yu DY (2004) Intraretinal oxygenation and oxygen consumption in the rabbit during systemic hyperoxia. Invest Ophthalmol Vis Sci 45:3223–3228
Dargel R (1992) Lipid peroxidation—a common pathogenetic mechanism? Exp Toxicol Pathol 44:169–181
Delori FC (1994) Spectrometer for noninvasive measurement of intrinsic fluorescence and reflectance of ocular fundus. Applied Optics 33:7439–7452
Delori FC, Dorey KC, Staurenghi G, Arend O, Goger DC, Weiter JJ (1995) In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol 36:718–729
Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, Salomon RG, Crabb JW, Anand-Apte B (2006) Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc Natl Acad Sci USA 103:13480–13484
Einbock W, Moessner A, Schnurrbusch UE, Holz FG, Wolf S (2005) Changes in fundus autofluorescence in patients with age-related maculopathy. Correlation to visual function: a prospective study. Graefes Arch Clin Exp Ophthalmol 243:300–305
Eldred GE, Lasky MR (1993) Retinal age-pigments generated by self-assembling lysomorphotropic detergents. Nature 361:724–726
Feeney-Burns L, Berman ER, Rothman H (1980) Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol 90:783–791
Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG (2003) Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem 278:42027–42035
Hammer M, Richter S, Guehrs KH, Schweitzer D (2006) Retinal pigment epithelium cell damage by A2-E and its photo-derivatives. Mol Vis 12:1348–1354
Haralampus-Grynaviski NM, Lamb LE, Clancy CM, Skumatz C, Burke JM, Sarna T, Simon JD (2003) Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc Natl Acad Sci USA 100:3179–3184
Haralampus-Grynaviski NM, Lamb LE, Simon JD, Krogmeier JR, Dunn RC, Pawlak A, Rozanowska M, Sarna T, Burke JM (2001) Probing the spatial dependence of the emission spectrum of single human retinal lipofuscin granules using near-field scanning optical microscopy. Photochem Photobiol 74:364–368
Holz FG, Bellman C, Staudt S, Schutt F, Volcker HE (2001) Fundus autofluorescence and development of geographic atrophy in age- related macular degeneration. Invest Ophthalmol Vis Sci 42:1051–1056
Hussain AA, Starita C, Marshall J (2003) Transport characteristics of ageing Bruch’s Membrane: Implications for age-related macular degeneration. In: Ioseliani OR (ed) Focus on macular degeneration research. Nova Biomedical Books, New York, pp 59–113
Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR (2005) Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J Biol Chem 280:39732–39739
Katz ML, Wendt KD, Sanders DN (2005) RPE65 gene mutation prevents development of autofluorescence in retinal pigment epithelial phagosomes. Mech Ageing Dev 126:513–521
Kobuch K, Herrmann AW, Framme C, Sachs HG, Gabel VP, Hillenkamp J (2008) Maintenance of adult porcine retina and retinal pigment epithelium in perfusion culture: characterisation of an organotypic in-vitro model. Exp Eye Res. DOI 10.1016/j.exer.2008.01.011
Kopitz J, Holz FG, Kaemmerer E, Schutt F (2004) Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie 86:825–831
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1:441–445
Marmorstein AD, Marmorstein LY, Sakaguchi H, Hollyfield JG (2002) Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol Vis Sci 43:2435–2441
Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH (2004) Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem 279:635–643
Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH (2001) Delayed dark-adaptation and lipofuscin accumulation in abcr /- mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci 42:1685–1690
Nilsson SE, Sundelin SP, Wihlmark U, Brunk UT (2003) Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol 106:13–16
Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J (1998) Isolation and one step preparation of A2-E and iso-A2-E fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA 95:14609–14613
Prince JH, Diesem CD, Eglitis I, Ruskell GL (1965) Anatomy and histology of the eye and orbit in domestic animals. Charles C. Thomas, Springfield
Radu RA, Mata NL, Bagla A, Travis GH (2004) Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc Natl Acad Sci USA 101:5928–5933
Radu RA, Mata NL, Nusinowitz S, Liu X, Travis GH (2004) Isotretinoin treatment inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Novartis Found Symp 255:51–63, discussion 63–7, 177–8
Rozanowska M, Pawlak A, Rozanowski B, Skumatz C, Zareba M, Boulton ME, Burke JM, Sarna T, Simon JD (2004) Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components. Invest Ophthalmol Vis Sci 45:1052–1060
Schütt F, Bergmann M, Holz FG, Kopitz J (2003) Proteins modified by Malondialdehyde, 4-Hydroxynonenal, or advanced glycation end products in Lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci 44:3663–3668
Schweitzer D, Hammer M, Schweitzer F, Anders R, Doebbecke T, Schenke S, Gaillard ER (2004) In vivo measurement of time-resolved autofluorescence at the human fundus. J Biomed Opt 9:1214–1222
Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D (1999) Characterization of peroxidized lipids in Bruch’s membrane. Retina 19:141–147
Sparrow JR, Boulton M (2005) RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res 80:595–606
Sparrow JR, Parish CA, Hashimoto M, Nakanishi K (1999) A2E, a Lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci 40:2988–2995
Starita C, Hussain AA, Pagliarini S, Marshall J (1996) Hydrodynamics of ageing Bruch’s membrane: implications for macular disease. Exp Eye Res 62:565–572
Strehl R, Schumacher K, Minuth WW (2004) Controlled respiratory gas delivery to embryonic renal epithelial explants in perfusion culture. Tissue Eng 10:1196–1203
Sundelin SP, Nilsson SE, Brunk UT (2001) Lipofuscin-formation in cultured retinal pigment epithelial cells is related to their melanin content. Free Radic Biol Med 30:74–81
von Rückmann A, Fitzke FW, Bird AC (1995) Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Brit J Ophthalmol 79:407–412
von Rückmann A, Fitzke FW, Bird AC (1997) Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci 38:478–486
Wassell J, Ellis S, Burke J, Boulton M (1998) Fluorescence properties of autofluorescent granules generated by cultured human RPE cells. Invest Ophthalmol Vis Sci 39:1487–1492
Wihlmark U, Wrigstad A, Roberg K, Nilsson SE, Brunk UT (1997) Lipofuscin accumulation in cultured retinal pigment epithelial cells causes enhanced sensitivity to blue light irradiation. Free Radic Biol Med 22:1229–1234
Acknowledgments
This work was supported by the German Ministry of Education and Research under grant No. 01EZ0309. The authors are grateful to Prof. Elizabeth Gaillard from Northern Illinois University for the gift of lipofuscin granules isolated from human donor eyes.
Conflict of interests
Hammer: none, Richter: none, Kobuch: none, Mata: employee of Sirion Therapeutics Inc., Schweitzer: none
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hammer, M., Richter, S., Kobuch, K. et al. Intrinsic tissue fluorescence in an organotypic perfusion culture of the porcine ocular fundus exposed to blue light and free radicals. Graefes Arch Clin Exp Ophthalmol 246, 979–988 (2008). https://doi.org/10.1007/s00417-008-0789-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0789-4