Abstract
Purpose
To evaluate the use of preoperative intravitreal bevacizumab (IVB) in patients undergoing pars plana vitrectomy (PPV) for complications of proliferative diabetic retinopathy (PDR).
Methods
We studied 22 patients with severe PDR. A preoperative complexity score (CS) was recorded. Eleven eyes were treated with IVB, 1.25 mg, 5–7 days before PPV (group 1), and 11 eyes underwent direct PPV (group 2). Surgical time and intra-operative manoeuvres were recorded. Main outcome measure was feasibility of surgery, secondary goal was the visual and anatomic outcome at 6 months.
Results
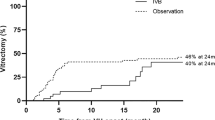
The average CS was 5.5, and was similar in the two groups. Mean surgical time was 57 minutes in group 1 vs 83 minutes in group 2; mean tool exchanges was 27 vs 53, intraoperative bleeding 5 vs 15, endodiathermy 2 vs 9. No complications were recorded after IVB. Mean pre-operative BCVA was 1.87 logMAR in group 1 and logMAR 2.04 in group 2. Mean pre-operative BCVA was 1.87 logMAR in the bevacizumab group and 2.04 logMAR in group 2, not significantly different (p = 0.7). Mean post-operative BCVA at 6 months was 0.88 logMAR in group 1 and logMAR 2.01 in control group 2, significantly different (p = 0.01). Post-operative BVCA improved in bevacizumab group from pre-operative value (p = 0.15), while in control group there was non-significant increase (p = 0.96). Anatomical attachment was achieved in 11 patients in group 1 vs nine patients in group 2.
Conclusions
IVB administered prior to vitrectomy was well tolerated and reduced active neovascularization, thus facilitating PPV.



Similar content being viewed by others
References
Varma R, Torres M, Pena F et al (2004) Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology 111:1298–1306
Kempen JH, O'Colmain BJ, Leske MC et al (2004) The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 122:552–563
Flynn HW Jr, Chew EY, Simons BD et al (1992) Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17.. Ophthalmology 99:1351–1357
Early Treatment Diabetic Retinopathy Study Research Group (1991) Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 98:823–833
Vander JF, Duker JS, Benson WE et al (1991) Long-term stability and visual outcome after favorable initial response of proliferative diabetic retinopathy to panretinal photocoagulation. Ophthalmology 98:1575–1579
Michaelson IC (1948) The mode of development the vascular system of the retina with some observation on its significance for certain retinal disorders. Trans Ophthalmol Soc UK 68:137–180
Aiello LP, Avery RL, Arrigg PG et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Medicine 331:1480–1487 Abstract
Aiello LP, Pierce EA, Foley ED et al (1995) Suppression of retinal neovascularization in vivo by inhibition of Vascular endothelial growth factor(VEGF)using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 92:10457–10461
Tolentino MJ, McLeod DS, Taomoto M et al (2002) Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol 133:373–385
Tolentino MJ, Miller JW, Gragoudas ES et al (1996) Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol 114:964–970
Tolentino MJ, Miller JW, Gragoudas ES et al (1996) Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103:1820–1828
Adamis AP, Shima DT, Tolentino MJ et al (1996) Inhibition of vascular endothelial growth factor prevents retinal ischemia associated iris neovascularization in a nonhuman primate. Arch Ophthalmol 114:66–71
Adamis AP, Miller JW, Bernal MT et al (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118:445–450
Yang JC, Haworth L, Sherry RM et al (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427–434
Adding a humanized antibody to vascular endothelial growth factor (bevacizumab, Bevacizumab) to chemotherapy improves survival in metastatic colorectal cancer. Clin Colorectal Cancer 8:85–88
Kabbinavar F, Hurwitz HI, Fehrenbacher L et al (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Yang JC (2004) Bevacizumab for patients with metastatic renal cancer: an update. Clin Cancer Res 10:S6367–S6370
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS (2005) Systemic bevacizumab (Bevacizumab) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112:1035–1047
Avery RL, Pieramici DJ, Rabena MD et al (2006) Intravitreal bevacizumab (Bevacizumab) for neovascular age-related macular degeneration. Ophthalmology 113:363–372
Rosenfeld PJ, Moshfeghi AA, Puliafito CA (2005) Optical coherence tomography findings after an intravitreal injection of bevacizumab (Bevacizumab) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 36:33133–33135
Nguyen QD, Shah S, Tatlipinar S et al (2005) Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 89:1368–1370
Rosenfeld PJ, Fung AE, Puliafito CA (2005) Optical coherence tomography findings after an intravitreal injection of bevacizumab (Bevacizumab) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 36:336–339
Iturralde D, Spaide RF, Meyerle CB et al (2006) Intravitreal bevacizumab (Bevacizumab) treatment of macular edema in central retinal vein occlusion: A short-term study. Retina 26:279–284
Spaide RF, Fisher YL (2006) Intravitreal bevacizumab (Bevacizumab) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 26:275–278
Oshima Y, Sakaguchi H, Gomi F, Tano Y (2006) Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol 142:155–158
Chen E, Park CH (2006) Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 26:699–700
Avery RL (2006) Regression of retinal and iris neovascularization after intravitreal bevacizumab (Bevacizumab) treatment. Retina 26:352–354
Avery RL, Pearlman J, Pieramici DJ et al (2006) Intravitreal bevacizumab (Bevacizumab) in the treatment of proliferative diabetic retinopathy. Ophthalmology 113:695–615
Rodrigo J, Costa R, Calucci D et al (2006) Intravitreal Bevacizumab (Bevacizumab) for persistent new vessels in diabetic retinopathy (IBEPE Study). Retina 26:1006–1013
Chobanian AV, Bakris GL, Black HR et al (2003) National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2572. Erratum in: JAMA 2003;290:197
Grigorian RA, Castellarin A, Fegan R et al (2003) Epiretinal membrane removal in diabetic eyes: comparison of viscodissection with conventional methods of membrane peeling. Br J Ophthalmol 87:737–741
Ferrara N (2004) Vascular endothelial growth factor: basic science in clinical progress. Endocr Rev 25:581–611
Mordenti J, Cuthberson RA, Ferrara N et al (1999) Comparisons of the intraocular tissue distribution pharmacokinetics and safety of 125-I-Labeled Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Patholol 27:536–544
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is original and has not been published previously.
The authors have no financial interests related to this publication and transfer copyright to the publisher upon acceptance.
Rights and permissions
About this article
Cite this article
Rizzo, S., Genovesi-Ebert, F., Di Bartolo, E. et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 246, 837–842 (2008). https://doi.org/10.1007/s00417-008-0774-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0774-y